- Department of Neurosurgery, University Clinics Schleswig-Holstein, Campus Kiel, Germany
- Department of Neurosurgery, University Clinic Essen, Campus Kiel, Germany
- Institute for Medical Informatics and Statistics, University Clinics Schleswig-Holstein, Campus Kiel, Germany
- Department of Neurosurgery, University Clinic Düsseldorf, Campus Kiel, Germany
Correspondence Address:
Alexandros Doukas
Department of Neurosurgery, University Clinics Schleswig-Holstein, Campus Kiel, Germany
DOI:10.4103/2152-7806.170246
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Doukas A, Maslehaty H, Barth H, Jürgen Hedderich, Petridis AK, Mehdorn HM. A novel simple measure correlates to the outcome in 57 patients with intracerebellar hematomas. Results of a retrospective analysis. Surg Neurol Int 23-Nov-2015;6:176
How to cite this URL: Doukas A, Maslehaty H, Barth H, Jürgen Hedderich, Petridis AK, Mehdorn HM. A novel simple measure correlates to the outcome in 57 patients with intracerebellar hematomas. Results of a retrospective analysis. Surg Neurol Int 23-Nov-2015;6:176. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-novel-simple-measure-correlates-to-the-outcome-in-57-patients/
Abstract
Background:The incidence of intracerebellar hemorrhages approaches 5–10% of all intracerebral hematomas. The clinical presentation varies from headaches and dizziness to rapid deterioration of consciousness to the point of coma in severe cases. In order to find some concrete criteria that could influence the prognosis of these patients, we performed this retrospective study.
Methods:We retrospectively analyzed the factors influencing the outcome of 57 patients with intracerebellar hematomas treated in our clinic in the last 7 years. The Glasgow Coma Scale (GCS) on admission, as well as other parameters as hypertension, diabetes mellitus, presence of malign tumors in the medical history, or the intake of anticoagulants were assessed as independent factors influencing the outcome of the patients. On the other hand, various computed tomography parameters on admission were also correlated with the clinical outcome such as, tight posterior fossa (TPF), volume of the hematoma, hydrocephalus, compression of the fourth ventricle, intraventricular bleeding, as well as the ratio of the maximal width of the hematoma in comparison to the width of the PF were taken into consideration.
Results:The results of the study showed that patients with poor GCS on admission had also a poor Glasgow Outcome Score. Interestingly there was a statistically significant correlation between the maximal width of the hematoma in comparison to the width of the PF and the outcome of the patients. It could be also shown that the patients with intraventricular hemorrhage, hydrocephalus, compression of the fourth ventricle over 50% of its maximal width and TPF, had a poor clinical outcome. Moreover, there was a statistically significant correlation of the volume of the hematoma and a poor clinical outcome.
Conclusions:We introduced as a new factor that is, the cerebellar hemorrhage/PF ratio and found out that the value >35% was associated to an unfavorable outcome.
Keywords: Intracerebellar hemorrhage, outcome, posterior fossa, risk factors, volume
INTRODUCTION
The incidence of spontaneous cerebellar hemorrhage (CBH) is determined with 5–10% of all intracerebral hemorrhages (ICHs).[
The clinical presentation of the patients varies. The initial symptoms include headache, nausea, vomiting, or dizziness or balance disturbance. The level of consciousness may also differ from awake and alert to stupor or even coma and sudden death due to brainstem compression and possible upward herniation.[
Brown-Sequard was the first one to describe this medical entity which was associated with poor outcome.[
Today, the indication and timing for surgical treatment of CBH is discussed controversially and is has to be considered in view of different parameters.[
Facing the controversies in the literature, we felt encouraged to review the data of patients with CBH, which were treated in our university clinic from 2005 to 2012. We determined clinical factors and computed tomography (CT) parameters and analyzed their influence on the clinical condition and outcome. Beside already described CT criteria, we introduce a novel attempt to analyze the size of hemorrhage compared to the space in the PF, to enable a customized evaluation.
METHODS
We analyzed the data of 57 patients with the diagnosis of CBH. All patients or their caregivers were informed about the acquisition of their data and gave their consent. Patients with trauma, hemorrhage in the brainstem, as well as patients with subarachnoid hemorrhage and AVM related hemorrhage were excluded from the study. In 8 patients the initial CT data could not be acquired.
The patients were divided into four groups according to the chosen treatment:
Conservative External ventricular drainage Surgical evacuation External ventricular drainage plus surgery.
After diagnosis of CBH via CT scanning, all patients were treated in the neurosurgical Intensive Care Unit (ICU) with continuous measurement of the arterial blood pressure, pulse rate, oxygen saturation, and continuous recording of electrocardiogram. The neurological condition in the continuing course was assessed by the physician at the ICU closely. Patients in a comatose state (Glasgow Coma Scale [GCS] <8) were sedated and received mechanical ventilation. If surgery was done, biopsy from the adjacent brain tissue was taken to enable histological work-up.
Considering a cerebrovascular risk-profile we analyzed co-morbidities and drug intake of the patients like arterial hypertension, anticoagulant therapy, presence of malignancies, previous ICH, vein thrombosis or lung embolisms, diabetes mellitus, and myocardial infarction (MI) or cerebral stroke in view of their influence on the disease pattern.
The GCS on admission was used to assess the initial clinical status. The patients were divided into the following groups for analysis: GCS 3–11 (poor condition) and GCS 12–15 (good condition). The Glasgow Outcome Score (GOS) and the modified Rankin scale (mRS) were used to assess the clinical outcome at time of discharge and 6 weeks follow-up. Since there was no statistically significant difference between the two scores regarding the outcome of the patients, the GOS was finally used in the statistical analysis. GOS 1–3 was determined as poor outcome and 4–5 as good outcome.
Multiple parameters of the initial CT scans were taken into consideration such as:
Total volume of the blood clot Presence of hydrocephalus Presence of tight PF (TPF) Intraventricular expansion of the hemorrhage Extent of compression of the fourth ventricle CBH/PF ratio: Ratio of the diameter of CBH in regards to the diameter of the PF Deep seated or superficial blood clot.
The volume of the blood clot was calculated according to the ABC/two rule.[
A new parameter was also taken into consideration: The CBH/PF ratio (given in percentages) describes the maximal width of the blood clot of a single slice in the initial CT scan opposed to the maximal width of the PF. In order to have the same landmarks in each patient, we used the distance between the points where the transverse sinus meets the sigmoid sinus on both sides as the reference point. The rationale for this procedure was to determine the size of CBH in relation to the size of the PF, to obtain adequate analysis for every patient. We assumed that the absolute size of CBH might not suitable enough for decision making for the treatment without analysis of the size of CBH and its relation to the available space in a personalized manner. To our knowledge this parameter was not yet described and analyzed in the literature.
Statistics
Statistical methods were used in an exploratory sense in this observational study on a selected clinical cohort. All calculations were performed with statistical software SPSS (V20) IBM Inc., IL, USA. For the univariate evaluation of correlations between the outcome parameters (dichotomized GOS and mRS) and selected prognostic factors the Chi-square test and Fisher's exact test were used. As a measure of prognostic relevance the odds ratios (ORs) (incl. 95% confidence interval [CI]) were also calculated. To adjust for associations between these factors, they were joined together in a multiple logistic regression model. Generally, statistical significance was determined as a P < 0.05.
RESULTS
The results of the study have been summarized in
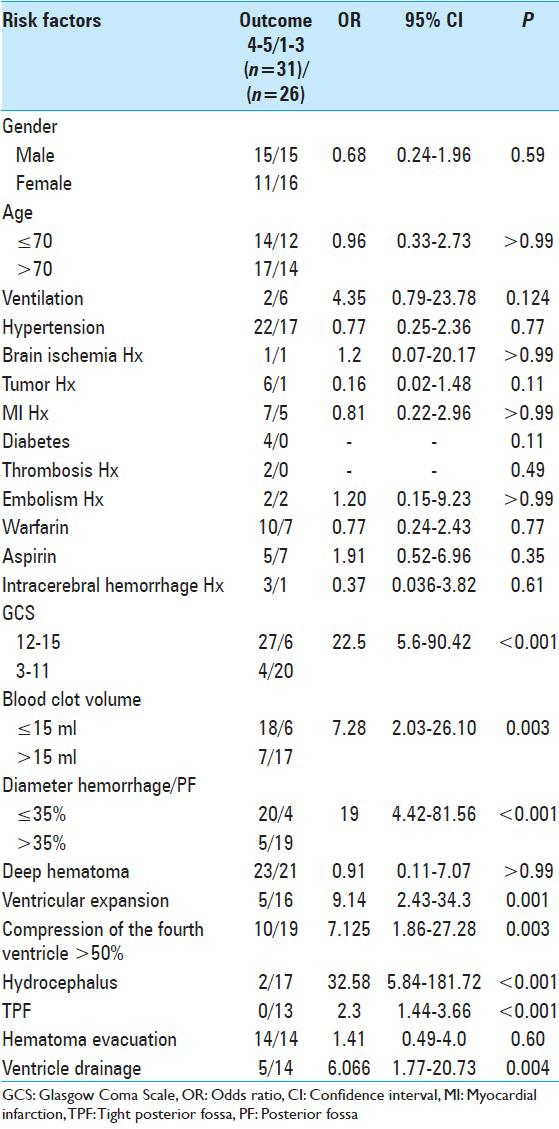
The mean age of the patients was 56 years (33–97 years). Thirty patients were male and 27 female (male/female ratio: 1.2). In total, 24 patients (42.1%) had a poor initial condition (GCS 3–11, 15 with GCS 3, 3 with GCS 5, 2 with GCS 7, 1 with GCS 9 and 3 with GCS 11). Thirty-three patients (57.9%) were in good clinical condition (GCS 13–15, 11 with GCS 13, 4 with GCS 14, and 18 with GCS 15).
Forty-four patients (77%) presented with deep seated hemorrhage which did not reach the surface of the cerebellum, whereby 5 patients (8.7%) had a hemorrhage which reached the dorsal margin of the cerebellum.
In 21 (36.8%) patients, the blood clot penetrated the fourth ventricle. In 28 cases, there was no intraventricular expansion of the hemorrhage.
In total, CBH reached the area of the fourth ventricle in 49 patients (85%) and compressed the fourth ventricle >50% in 29 patients (59.1%) and in 20 patients (40.8%) <50%.
In total, 17 of these patients underwent blood clot evacuation, 8 received external ventricular drainage and 10 both evacuation and drainage. Twenty-two patients were treated conservatively.
Thirteen patients (22.8%) fulfilled the necessary criteria of TPF. Nineteen patients (33.3%) developed consecutive hydrocephalus, whereas 30 patients did not suffer from hydrocephalus. The GOS of the patients was as follows: 10 patients died (total mortality 17.5%); 4 patients (7%) had a GOS 2, 12 patients (21%) GOS 3, 19 patients (33.3%) had a GOS 4, and 12 patients (21%) had a GOS 5. The assessment was done at time of discharge and at 6 weeks follow-up.
Considering the medical history of the patients, 7 (12.8%) had a malignant tumor and in 1 patient, craniotomy revealed a metastasis of a previously unknown tumor. Four of the patients (7%) experienced an ICH in their history. Twelve patients (21%) had a MI in their previous history, 4 (7%) had diabetes mellitus. Two (3.5%) had deep vein thrombosis and 4 (7%) lung embolism. In total, 29 patients (50.1%) had anticoagulant therapy. Twelve patients (21.1%) had aspirin and 17 (30%) coumarine as standard medication. Two patients (3.5%) had both, whereas further 2 patients (3.5%) had clopidogrel and aspirin. Thirty-nine patients (68.4%) had arterial hypertension, which was also the main cause of the hemorrhage.
The highest volume was 75.3 ml and the lowest 0.3 ml. The mean volume was 19.74 ml.
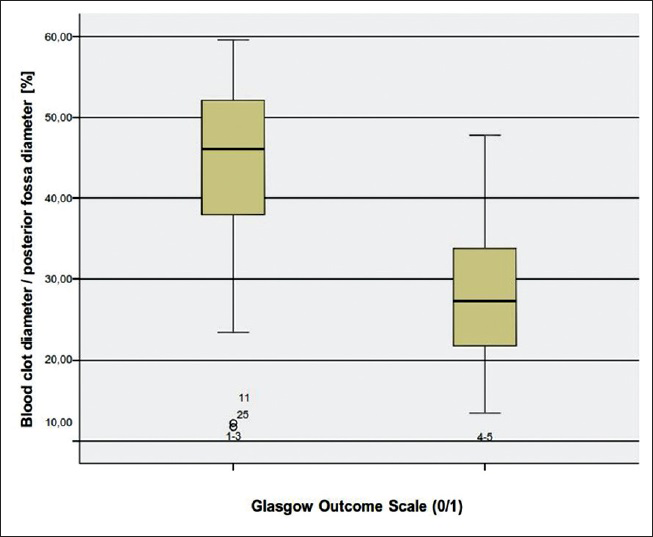
Regarding the CBH/PF ratio, the highest value was 59.6% and the lowest 11.7%. The mean value was 35.4%.
Although it seems incompatible at first, the reason very small hematomas had a phenomenally high percentage of CBH/PF ratio was that they were horizontally configured. So the ratio that was measured according to the parallel line of the maximum width of the PF was larger. Another reason was often the atrophy of the cerebellum in older patients which resulted in a higher CBH/PF ratio.
Statistical analysis
The age, as well as the gender of the patients did not influence the outcome significantly. This was also shown for the preexisting medical conditions such as hypertension, diabetes mellitus, tumor, lung embolism, previous ICH, MI, or cerebral stroke. Interestingly, our data did not reveal a significant correlation of poor outcome and the use of anticoagulants such as aspirin or coumarine. Of the 19 patients, who received ventricular drainage, 14 had a GOS of 1–3 and 5 a GOS of 4–5. This could be explained through the fact that the patients with drainage presented with hydrocephalus, which was an independent factor for worse outcome. A statistically significant difference between patients with evacuation of the blood clot and without operation could not be demonstrated.
The presence of intraventricular bleeding correlated significantly with the outcome. Five of the patients with intraventricular bleeding had a GOS of 4–5, whereas 16 had a poor outcome (GOS 1–3; P < 0.001; OR: 9.143).
Presence of hydrocephalus on admission was the most significant factor for poor outcome. Only 8% of the patients who had a GOS of 4–5 had also hydrocephalus on admission, whereas 73.91% of the patients with worse outcome that is, GOS 1–3, had hydrocephalus on initial presentation (P < 0.0001; OR: 32.583). External ventricular drainage was shown to play a negative role regarding the outcome of the patients (P = 0.04, OR: 6.067). This observation was obviously associated with the presence of hydrocephalus which, as we mentioned, was the most influencing factor for an unfavorable outcome. 83.87% of the patients who had a good outcome score (GOS 4–5) did not receive an external ventricular drainage since they did not have hydrocephalus on initial appearance.
As already in other studies documented[
Whether the blood clot reached the superficial layer of the cerebellum or not did not play a significant role regarding the outcome (P = 0.931).
The compression of the fourth ventricle was also associated with the poor outcome when it exceeded more than the half of its diameter. We found only 10 patients with compression of the fourth ventricle <50%. These patients had a GOS of 4–5. On the other hand, 19 patients who had a more than 50% obliteration of the fourth ventricle had a worse outcome (GOS 1–3). There was a statistically significant difference (P < 0.003; OR: 7.125).
The presence of TPF has been reported to negatively influence the outcome of the patients.[
Regarding the duration of mechanical ventilation more than 48 h, we found no significant correlation with the outcome.
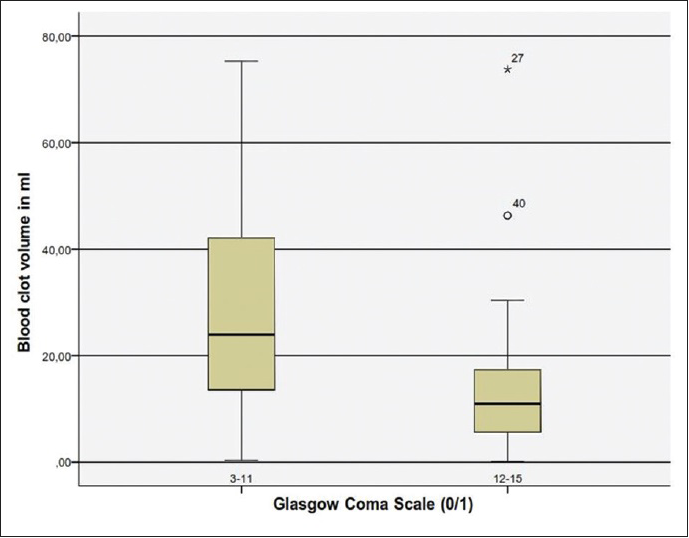
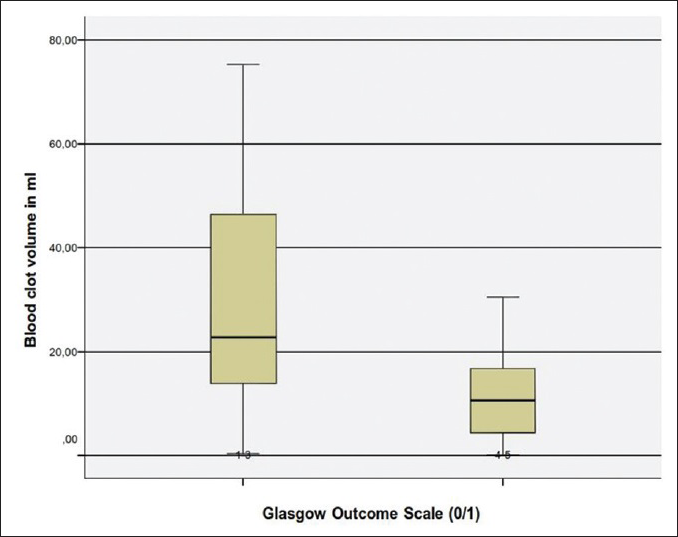
Concerning the blood volume, our analysis revealed that blood clot volume over 15 ml was associated to a significant poorer outcome (GOS 1–3) [
The cerebellar blood hemorrhage/posterior fossa ratio
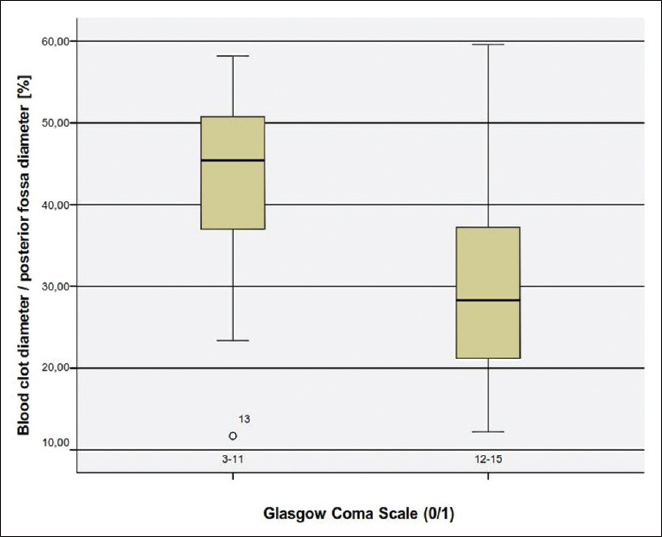
We examined another CT parameter that is, the cerebellar blood hemorrhage/PF ratio. To our best of knowledge this parameter has never been previously considered. We measured the maximal width of the blood clot in regards to the maximal width of the PF. In order to make a comparison feasible we considered the maximal diameter of the PF as the distance between the points where the transverse sinus meets the sigmoid sinus on both sides. We assessed then in percentage the ratio in each patient and made a univariate statistical analysis. Concerning the CBH/PF ratio, there was a statistically significant correlation to the outcome. The higher the CBH/PF ratio was, the poorer was the outcome [
All the parameters that were assessed in regards to the GOS are shown in
DISCUSSION
Since the first surgical evacuation of CBH in 1906 by Balance[
From the patients who had a GOS of 1–3, 20 had an initial GCS of 3–11 (P < 0.001, OR: 22.5, CI: 95%). On the other hand, 27 patients who had a GCS of 12–15, had a GOS of 4–5 (P < 0.001, OR: 3.774, CI: 95%). This was also shown in older studies such as the one of Dunne et al. in 1987. The authors postulated a poor outcome in patients with either initial presentation or later development of stupor or coma.[
Wu et al. studied the predictors of 1-week mortality in patients with CBH. Two factors were found to strongly influence the mortality: GCS <8 and brainstem compression.[
On the other hand, patients with a GCS >13 and hematoma of maximum diameter of 40 mm or less has been suggested to be treated conservatively.[
In regards to the operative treatment many options have been proposed. The role of surgery is to reduce the mass effect and the intracranial pressure. As a result, the local blood flow is improved and the breakdown products such as free radicals are minimized.[
Since the first endoscopic surgical evacuation of an intracerebral hematoma by Auer et al. in 1984, this technique is also discussed as treatment option for CBH.[
In conclusion, our analysis have shown that the most significant factors for poor outcome are poor GCS grade at admission, presence of hydrocephalus, which can be associated to intraventricular hemorrhage or compression of the four ventricle, total size of CBH >15 ml, presence of TPF and a CBH/PF ratio above 35%.
To the best of our knowledge, the CBH/PF ratio has not been analyzed so far. This ratio seems to be a reliable marker, which needs to be proven in prospective studies. The CBH/PF ratio is easy to calculate and might be more accurate than the total size of the hemorrhage alone. As an additional marker the CBH/PF ratio might be useful for decision making between surgery and conservative management of CBH in regard to the clinical condition of the patients. We believe that this new parameter could determine another way of approaching patients with intracerebellar hematomas that is, not only considering the volume but also the configuration of the hemorrhage, since a rather smaller hemorrhage which extends horizontally could have a high CBH/PF ratio and thus lead to a worse prognosis, especially in older patients with a given cerebellar atrophy.
Limitations of the study
The study was a nonrandomized retrospective study with the analogous limitations. We think however, that through a thorough statistical analysis we could narrow down the factors which influence the clinical outcome. Another limiting factor was the rather small number of the patients which is nevertheless similar to other retrospective studies. Randomized prospective studies with larger cohorts should be performed in the future in order to come to more reliable conclusions.
CONCLUSION
Our study suggests a significant correlation of the initial clinical status that is, GCS with the outcome (GOS). Patients with poor clinical condition on admission had a poor outcome whether treated operatively or conservatively. We also found that patients with a volume of blood clot above 15 ml also had an unfavorable outcome. The volume of the clot affected also their initial clinical presentation. Moreover, we found a strong correlation between hydrocephalus and poor GOS. Hydrocephalus was statistically the most influential factor in regards to the outcome. Other factors contributing to worse outcome was the presence of TPF, intraventricular bleeding and compression of the fourth ventricle over 50%. We introduced as a new factor that is, the CBH/PF ratio and found out that the value >35% was associated to an unfavorable outcome. Our study has its limitations which include the small number of patients and its retrospective character. Nevertheless, we strongly hope to have added some more insight to this controversial disease pattern.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg. 1989. 70: 530-5
2. Ballance H. A case of traumatic haemorrhage into the left lateral lobe of the cerebellum treated by operation with recovery. Surg Gynecol Obstet. 1906. 3: 223-5
3. Cohen ZR, Ram Z, Knoller N, Peles E, Hadani M. Management and outcome of non-traumatic cerebellar haemorrhage. Cerebrovasc Dis. 2002. 14: 207-13
4. Da Pian R, Bazzan A, Pasqualin A. Surgical versus medical treatment of spontaneous posterior fossa haematomas: A cooperative study on 205 cases. Neurol Res. 1984. 6: 145-51
5. Dahdaleh NS, Dlouhy BJ, Viljoen SV, Capuano AW, Kung DK, Torner JC. Clinical and radiographic predictors of neurological outcome following posterior fossa decompression for spontaneous cerebellar hemorrhage. J Clin Neurosci. 2012. 19: 1236-41
6. Dammann P, Asgari S, Bassiouni H, Gasser T, Panagiotopoulos V, Gizewski ER. Spontaneous cerebellar hemorrhage – Experience with 57 surgically treated patients and review of the literature. Neurosurg Rev. 2011. 34: 77-86
7. Dinsdale HB. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol. 1964. 10: 200-17
8. Dolderer S, Kallenberg K, Aschoff A, Schwab S, Schwarz S. Long-term outcome after spontaneous cerebellar haemorrhage. Eur Neurol. 2004. 52: 112-9
9. Donauer E, Loew F, Faubert C, Alesch F, Schaan M. Prognostic factors in the treatment of cerebellar haemorrhage. Acta Neurochir (Wien). 1994. 131: 59-66
10. Dunne JW, Chakera T, Kermode S. Cerebellar haemorrhage - Diagnosis and treatment: A study of 75 consecutive cases. Q J Med. 1987. 64: 739-54
11. Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008. 7: 951-64
12. Heros RC. Cerebellar hemorrhage and infarction. Stroke. 1982. 13: 106-9
13. Jüttler E, Steiner T. Treatment and prevention of spontaneous intracerebral hemorrhage: Comparison of EUSI and AHA/ASA recommendations. Expert Rev Neurother. 2007. 7: 1401-16
14. Kobayashi S, Sato A, Kageyama Y, Nakamura H, Watanabe Y, Yamaura A. Treatment of hypertensive cerebellar hemorrhage - Surgical or conservative management?. Neurosurgery. 1994. 34: 246-50
15. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
16. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011. 20: 208-13
17. Nakano T, Ohkuma H, Ebina K, Suzuki S. Neuroendoscopic surgery for intracerebral haemorrhage - Comparison with traditional therapies. Minim Invasive Neurosurg. 2003. 46: 278-83
18. Ott KH, Kase CS, Ojemann RG, Mohr JP. Cerebellar hemorrhage: Diagnosis and treatment. A review of 56 cases. Arch Neurol. 1974. 31: 160-7
19. Pearce JM. Brown-Séquard's description of spontaneous cerebellar haemorrhage. J Neurol Neurosurg Psychiatry. 1988. 51: 634-
20. Pollak L, Rabey JM, Gur R, Schiffer J. Indication to surgical management of cerebellar hemorrhage. Clin Neurol Neurosurg. 1998. 100: 99-103
21. Salvati M, Cervoni L, Raco A, Delfini R. Spontaneous cerebellar hemorrhage: Clinical remarks on 50 cases. Surg Neurol. 2001. 55: 156-61
22. Tai-Ngar L, Fairholm DJ, Shu TF, Chang CN, Lee ST, Chen HR. Surgical treatment of spontaneous cerebellar hemorrhage. Surg Neurol. 1985. 23: 555-8
23. Weisberg LA. Acute cerebellar hemorrhage and CT evidence of tight posterior fossa. Neurology. 1986. 36: 858-60
24. Wu YT, Li TY, Chiang SL, Chu HY, Chang ST, Chen LC. Predictors of first-week mortality in patients with acute spontaneous cerebellar hemorrhage. Cerebellum. 2013. 12: 165-70