- Division of Neurosurgery, University of Arizona, Tucson, Arizona, USA
- Valley Fever Center for Excellence, University of Arizona Medical Center, University of Arizona College of Medicine, Tucson, Arizona, USA
Correspondence Address:
Ali A. Baaj
Division of Neurosurgery, University of Arizona, Tucson, Arizona, USA
DOI:10.4103/2152-7806.158979
Copyright: © 2015 Martirosyan NL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Martirosyan NL, Skoch JM, Zaninovich O, Zoccali C, Galgiani JN, Baaj AA. A paradigm for the evaluation and management of spinal coccidioidomycosis. Surg Neurol Int 17-Jun-2015;6:107
How to cite this URL: Martirosyan NL, Skoch JM, Zaninovich O, Zoccali C, Galgiani JN, Baaj AA. A paradigm for the evaluation and management of spinal coccidioidomycosis. Surg Neurol Int 17-Jun-2015;6:107. Available from: http://surgicalneurologyint.com/surgicalint_articles/paradigm-evaluation-management-spinal/
Abstract
Background:Coccidioidomycosis is a fungal infection that is endemic to parts of the Southwestern United States. When infection involves the spine, the treatment strategies can be challenging. We have devised a management protocol for spinal coccidioidomycosis based on a review of the literature and our experience.
Methods:The electronic literature search of National Library of Medicine for publications from 1964 to 2014 was performed using the following keywords: Coccidioidomycosis and spine. The search yielded 24 papers. Treatment strategies were summarized into a treatment protocol.
Results:A total of 164 cases of spinal coccidioidomycosis were identified, ranging in age from 80 years. Males (n = 131) and African-Americans (n = 79) were strikingly over-represented. Medical therapy: Once a diagnosis of spinal coccidioidomycosis is established, antifungal therapy should always be started. Antifungal therapy with amphotericin B or azoles like fluconazole. Medical therapy needs to be continued for many years and sometimes indefinitely to reduce disease recurrence or progression. Surgical management is indicated in cases with mechanical instability, neurologic deficit, medically intractable pain, or progression of infection despite antifungal therapy.
Conclusions:This work provides a working protocol involving assessment and reassessment for the management of spinal coccidioidomycosis. Medical management with antifungal agents in some cases can provide satisfactory disease control. However, in patients with mechanical instability, neurologic deficit, medically intractable pain or disease progression disease control may only be achieved with surgical debridement and stabilization.
Keywords: Fungal infection, osteomyelitis, spinal coccidioidomycosis, spinal fusion, spine, stabilization, vertebral
INTRODUCTION
Coccidioidomycosis, also known as San Joaquin Valley fever, is a fungal infection that is caused by Coccidioides spp. Infection is initiated by the inhalation of spores that originate in endemic soils within the Southwestern United States, Mexico, and Central and South America.[
Although 60% of infections are subclinical and self-resolving, 1% of them or approximately 5% of all diagnosed infections, result in hematogenous extra-pulmonary dissemination and the attendant increase in morbidity and risk of death.[
MATERIALS AND METHODS
The electronic literature search of National Library of Medicine for publications from 1964 to 2014 was performed using following keywords: Coccidioidomycosis and spine. The articles were reviewed using the following criteria for inclusion: Spinal coccidioidomycosis, surgical and medical management. The cases from all of the citations were considered because of the infrequent occurrence of this pathology.
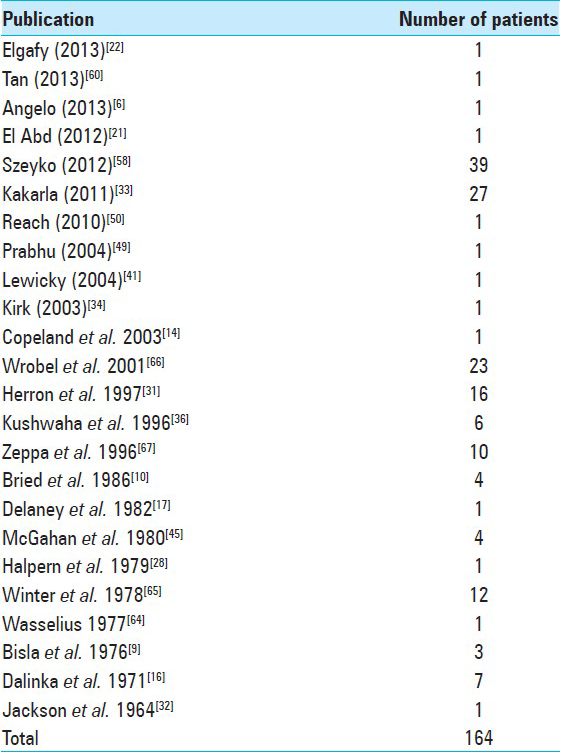
The search was limited to the English language and yielded 21 papers. Bibliographies of publications were screened for additional citations. A total of 24 papers were selected (13 case reports and 11 case series) [
Epidemiological characteristics (age, gender, race), clinical findings (comorbidities, segments involved, symptoms), treatment approach, and clinical outcomes were analyzed. Treatment strategies were summarized into the treatment protocol.
RESULTS
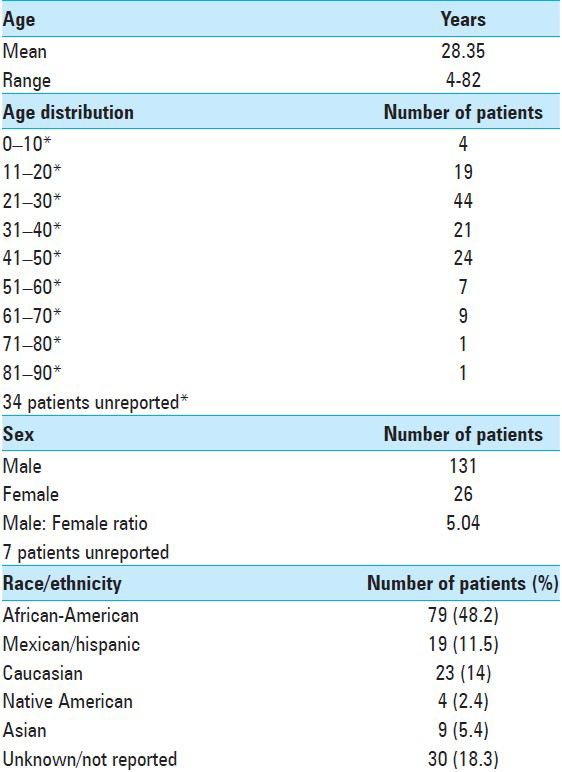
A total of 164 cases of spine coccidioidomycosis were identified (131 male and 26 female). Of these, 43 were derived from our institution (Szeyko, Bried). Their mean age at presentation was 28.3 years (range: 4–82 years). Most affected patients were in their third decade of life. Men were strikingly over-represented with this complication occurring more frequently than in women. Furthermore, nearly one-half of all infections occurred in African-Americans (48.5%) [
Unfortunately, in only 28 patients was the precise method of diagnosis indicated: Serologic testing in 17 and computed tomographic (CT) guided biopsy in 11. If the cerebrospinal fluid analysis demonstrates an eosinophilic pleocytosis, this can be highly supportive to a Coccidioides meningitis or parameningeal infection.
Spinal levels
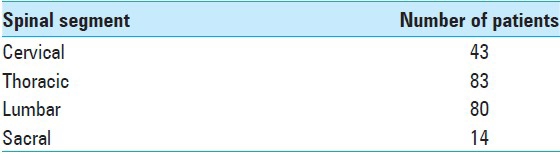
The thoracic and lumbar vertebras were the more commonly involved, followed by cervical spine and sacrum (83, 80, 43, and 14 cases, respectively) [
Symptoms
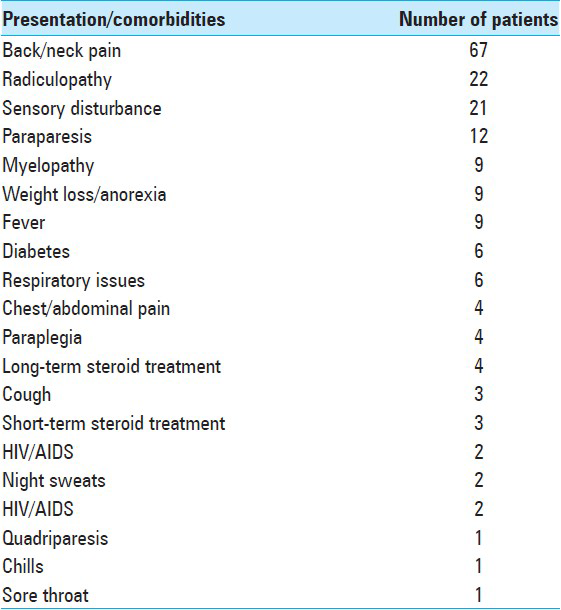
About 40.8% of patients presented with local pain, and 42% had neurologic symptoms [
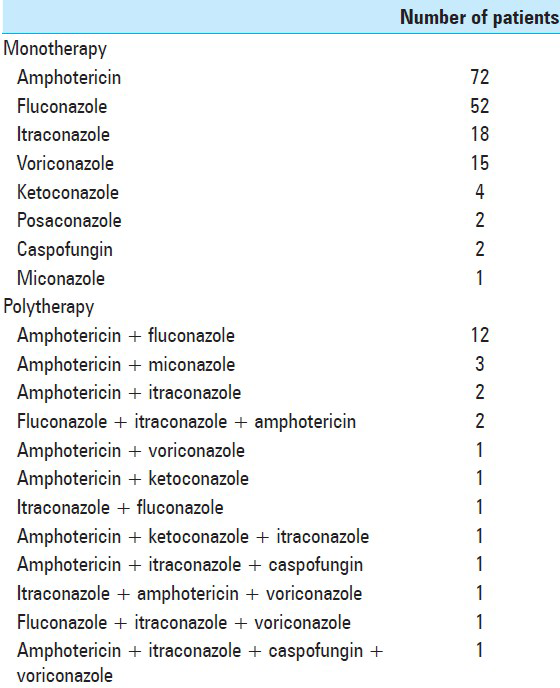
Medical therapy
Antifungal therapy included administration of amphotericin B combined or followed by a triazole. In most of the reported cases, medical therapy was continued indefinitely to reduce disease recurrence [
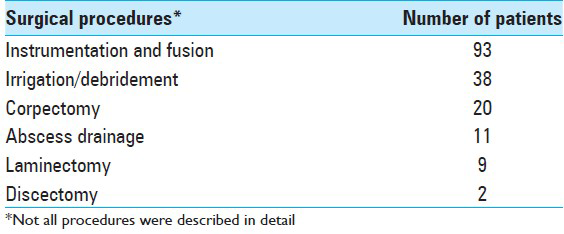
Surgery
In patients with mechanical instability, neurologic deficit, medically intractable pain or disease progression surgical debridement, decompression of neural elements and stabilization was often necessary. 117 patients underwent surgical intervention [
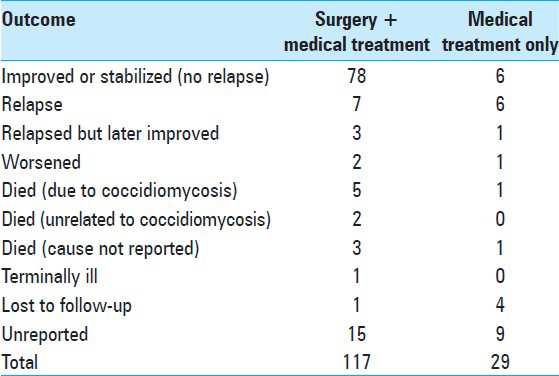
Outcomes
From 117 patients who underwent surgical intervention, only 102 had reported the clinical outcome. From those, 76.4% patients did not have a recurrence. 4.9% patient died due to the progression of disease despite treatment. 18 patients did not have reported treatment modalities and outcome [
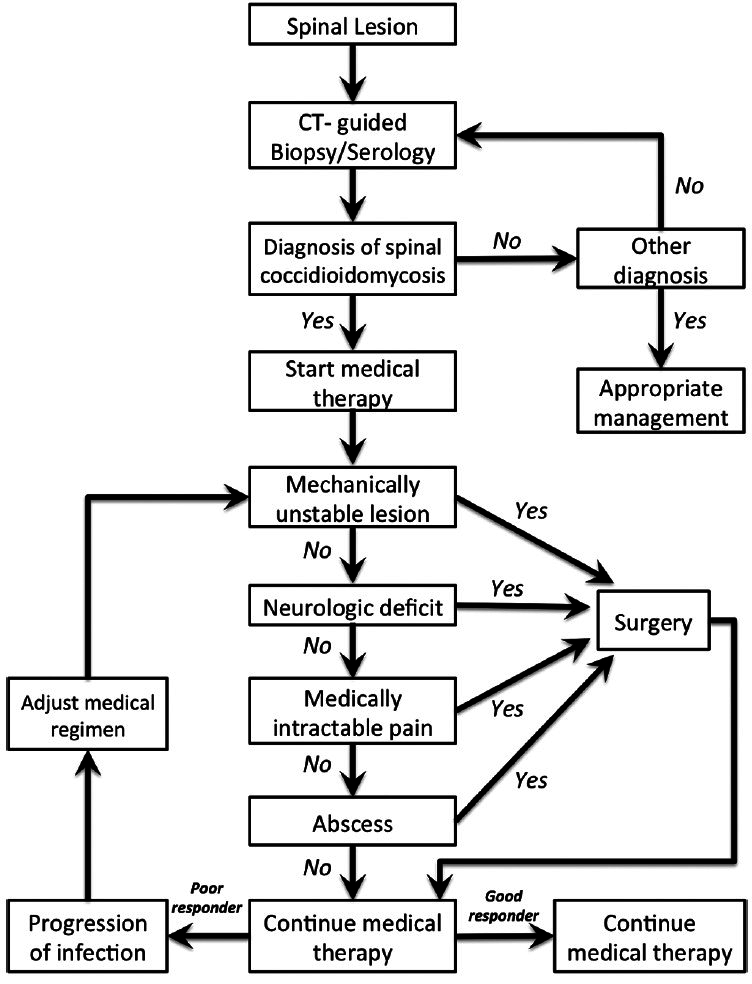
SURGICAL PROTOCOL
Patients with elevated specific antibodies against Coccidioides and confirmed with imaging spinal lesion require a CT-guided biopsy to rule out concurrent spinal tumor. Patients start antifungal therapy after diagnosis confirmation by histology and cultures. The early progression of disease does not necessarily indicate failure of medical treatment. Lesions can evolve with medical therapy even if fungal proliferation has been blocked. This is because of the persistence of pro-inflammatory fungal debris in the lesion. This is especially true if some lesions are stable or healing and one or more are worsening. The fact that surgery is added is not necessarily a reason to replace antifungal medication. It is imperative to assess the mechanical stability of affected spine, as well as the presence of neurologic deficit. Patients with acute neurologic deficit require emergency decompressive surgery, whereas mechanical instability and intractable pain can be addressed with an elective procedure. Goals for surgical intervention are decompression and stabilization. Follow-up imaging is critical to assess the effectiveness of treatment and disease progression. Magnetic resonance imaging (MRI) with and without gadolinium contrast can be obtained in 1, 3, and 6 months postoperatively or sooner if symptoms continue to progress.
ILLUSTRATIVE CASES
Case 1
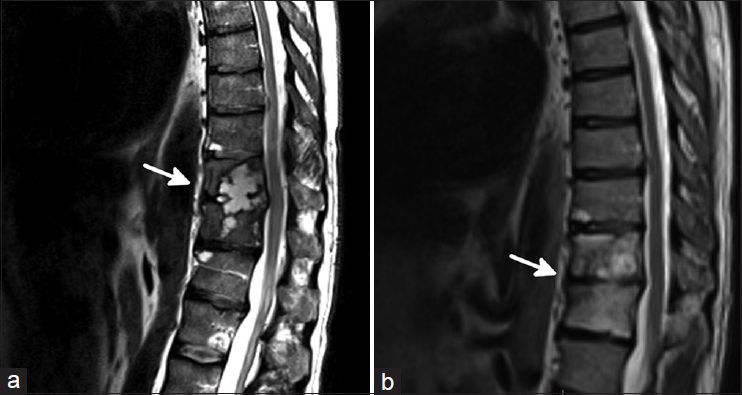
Forty-six-year-old Caucasian male, with past medical history of coccidioidal pneumonia treated for several months with fluconazole 6 years earlier, presented with a 3-month history of progressive mid back pain radiating to flank bilaterally. Symptoms were worse at night. The patient also had generalized joint pain, fatigue, weight loss. Physical examination did not reveal focal neurological deficit. MRI spine showed cystic lesions in T11/12 with vertebral body and disc space involvement, and minimal epidural involvement [
Case 2
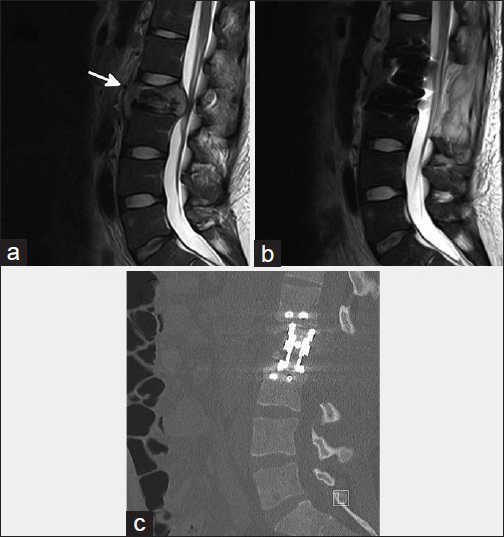
Twenty-two-year-old African-American male with no significant past medical history presented to emergency department with 3 months history of low back pain. Imaging studies revealed L1 lytic lesion. Although CT guided biopsy was negative for cocci, serologic studies were positive for cocci IgG. Patient was started with fluconazole and analgesics. Despite medical treatment patient's condition continued to worsen. He presented again after 4 weeks of medical therapy. He developed medically intractable low back pain and new onset bilateral lower extremity paresthesia. MRI showed a new L2 epidural infection with lumbar stenosis [
Figure 3
Sagittal magnetic resonance imaging reconstructions show L2 spinal coccidioidomycosis (arrow) at the presentation with severe spinal canal narrowing (a) and after surgical decompression of neural elements (b). Postoperative sagittal computed tomographic reconstruction shows L2 corpectomy (c)
DISCUSSION
Spinal coccidioidomycosis is a rare disease and associated with diagnostic and treatment challenges. In this review, we attempted to summarize existing knowledge about the nature of this disease. Furthermore, we devised detailed management protocol of spinal coccidioidomycosis.
Vertebral infection appears to be more prevalent in certain populations. Residents, former residents, or people who have traveled to endemic areas within the last 2 years are almost prerequisite history. However, in patients with prior pulmonary coccidioidomycosis who received antifungal therapy for this can have a longer interval between initial infection and their presentation of vertebral dissemination as illustrated in Case 1 above. We found that the vast majority of patients reported in the literature were male. Vertebral infections were also more in African-American patients. While immunocompromised patients, such as those taking immunosuppressant drugs (especially chronic corticosteroid use), those positive for HIV, pregnant women, and infants under the age of 1, are potentially at an increased risk of dissemination, it is critical to note that many patients with vertebral infection, if not the majority, were not immunocompromised.[
Symptomatic patients with coccidioidomycosis commonly typically present with symptoms of primary lung infection.[
Diagnosing vertebral coccidioidomycosis can be complicated but should be done as quickly as possible to prevent the infection from expanding and potentially worsening the patient's clinical condition. The inflammatory marker C-reactive protein and the erythrocyte sedimentation rate, while frequently elevated, are not specific for vertebral coccidioidomycosis, showing abnormal results for a variety of vertebral infections.[
Imaging can expose erosive defects and/or sclerosis in vertebrae, vertebral endplates, or disks, as well as osteopenia, vertebral body collapse or paraspinal extension.[
Diagnosis requires biopsy samples that either reveal Coccidioides posadasii/immitis spherules by microscopy or yield growth in culture. We recommend obtaining a tissue diagnosis unless the risk of the procedure is prohibitive. This is true even for patients who present with vertebral disease and coccidioidomycosis in other organ systems since the vertebral disease could have an alternate cause. Currently, obtaining a sample for pathogen identification can be done effectively by CT-guided biopsies.[
Management of vertebral coccidioidomycosis has most frequently involved both pharmacological treatments and surgery. Of the reported cases we found, 3 of the 4 patients who did not undergo medical treatment died of sepsis (the other was lost to follow-up). All patients require medical therapy or risk progressive morbidity and eventual death. We found that historically, the various forms of amphotericin have been the standard of care. However, unless the vertebral lesions are very extensive and potentially unstable, the current medical approach has shifted to beginning treatment with azole antifungals for a minimum of 12–18 months after the patient begins to show evidence of response to treatment.[
We found that fluconazole was the most frequently reported antifungal used for therapy, followed distantly by voriconazole and itraconazole. In a comparison of fluconazole 400 mg/day and itraconazole 200 mg BID for progress coccidioidal infections, the subset with skeletal infections were twice as likely to respond to itraconazole.[
Amphotericin or polytherapy, which usually included amphotericin, was the next step for patients who responded poorly to azoles alone. A substantial number of reported patients underwent polytherapy, often due to resistance to monotherapy.[
Repeated imaging should be used at regularly scheduled follow-ups to monitor lesions for stabilization, improvement or deterioration. Understanding this and communicating it to the patient is critically important to optimizing management. We found many that many patients were lost to follow-up, and also that poor compliance with the medical regimen was a major cause of relapse or clinical decline.[
In addition to pharmacological therapy, the vast majority of reported patients have also required and undergone surgical intervention. It can be difficult to determine when surgery is necessary, but generally the decision rests on the severity of the disease.[
Although medical hardware has been associated with biofilm production and infection, studies using metal hardware in spine surgery have also demonstrated infection eradication in most, and frequently all, study subjects.[
Patients who are have resided in or traveled to endemic areas and present with flu-like symptoms, back pain, progressive extremity weakness, or other neurologic symptoms in the extremities should raise suspicion of vertebral coccidioidomycosis. These patients should receive a thorough work-up that likely includes MRI, especially in cases involving progressing neurological symptoms. Although MRI cannot be used for diagnosis, it is the most sensitive method of detecting vertebral lesions and aids in planning biopsies and surgeries. Patients diagnosed with vertebral coccidioidomycosis require long-term pharmacological treatment and may require surgery. Finally, it is imperative to educate patients about the seriousness of the disease to encourage them to adhere to their treatment regimen and follow-up regularly for disease monitoring.
CONCLUSIONS
This work provides a working protocol for the management of spinal coccidioidomycosis. Medical management with antifungal agents can provide satisfactory disease control. However, in patients with mechanical instability, neurologic deficit, medically intractable pain or disease progression surgical debridement and stabilization is often necessary.
References
1. Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009. 122: 770-77
2. Ahariz M, Courtois P. Candida albicans biofilm on titanium: Effect of peroxidase precoating. Med Devices (Auckl). 2010. 3: 33-40
3. Akers KS, Mende K, Cheatle KA, Zera WC, Yu X, Beckius ML. Biofilms and persistent wound infections in United States military trauma patients: A case-control analysis. BMC Infect Dis. 2014. 14: 190-
4. Ampel NM. Coccidioidomycosis: A review of recent advances. Clin Chest Med. 2009. 30: 241-251
5. Ampel NM. New perspectives on coccidioidomycosis. Proc Am Thorac Soc. 2010. 7: 181-85
6. Angelo KM, Nnedu ON. Rare manifestations of coccidioidomycosis. J La State Med Soc. 2013. 165: 137-39
7. Anstead GM, Corcoran G, Lewis J, Berg D, Graybill JR. Refractory coccidioidomycosis treated with posaconazole. Clin Infect Dis. 2005. 40: 1770-76
8. Bergstrom L, Yocum DE, Ampel NM, Villanueva I, Lisse J, Gluck O. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004. 50: 1959-66
9. Bisla RS, Taber TH. Coccidioidomycosis of bone and joints. Clin Orthop Relat Res. 1976. 121: 196-204
10. Bried JM, Galgiani JN. Coccidioides immitis infections in bones and joints. Clin Orthop Relat Res. 1986. 211: 235-43
11. Buchelt M, Lack W, Kutschera HP, Katterschafka T, Kiss H, Schneider B. Comparison of tuberculous and pyogenic spondylitis. An analysis of 122 cases. Clin Orthop Relat Res. 1993. 296: 192-99
12. Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976). 2006. 31: 2695-700
13. Catanzaro A, Cloud GA, Stevens DA, Levine BE, Williams PL, Johnson RH. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin Infect Dis. 2007. 45: 562-68
14. Copeland B, White D, Buenting J. Coccidioidomycosis of the head and neck. The Annals of otology, rhinology, and laryngology. 2003. 112: 98-101
15. Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992. 51: 690-97
16. Dalinka MK, Greendyke WH. The spinal manifestations of coccidioidomycosis. Journal of the Canadian Association of Radiologists. 1971. 22: 93-9
17. Delaney P, Niemann B. Spinal cord compression by Coccidioides immitis abscess. Archives of neurology. 1982. 39: 255-56
18. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006. 55: 929-942
19. Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001. 33: 1387-92
20. Donlan RM. Biofilms and device-associated infections. Emerging infectious diseases. 2001. 7: 277-81
21. El Abd OH, Fusco HN, Gomba L, Lew M, Jenis L. Coccidioidomycosis infection presenting with thoracic spinal pain. PM R. 2012. 4: 450-55
22. Elgafy H, Miller J, Meyers S, Assaly R. Disseminated coccidioidomycosis of the spine. Spine J. 2013. p.
23. Erturer E, Tezer M, Aydogan M, Mirzanlı C, Ozturk I. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010. 19: 2209-15
24. Fu TS, Yang SC, Tsai TT, Chen LH, Lai PL, Niu CC. Percutaneous endoscopic debridement and drainage in immunocompromised patients with complicated infectious spondylitis. Minim Invasive Ther Allied Technol. 2010. 19: 42-47
25. Galgiani JN, Ampel NM, Catanzaro A, Johnson RH, Stevens DA, Williams PL. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000. 30: 658-61
26. Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med. 2000. 133: 676-86
27. Haghighi F, Mohammadi SR, Mohammadi P, Eskandari M, Hosseinkhani S. The evaluation of Candida albicans biofilms formation on silicone catheter, PVC and glass coated with titanium dioxide nanoparticles by XTT method and ATPase assay. Bratisl Lek Listy. 2012. 113: 707-11
28. Halpern AA, Rinsky LA, Fountain S, Nagel DA. Coccidioidomycosis of the spine: unusual roentgenographic presentation. Clin Orthop Relat Res. 1979. 140: 78-79
29. Halpern EM, Bacon SA, Kitagawa T, Lewis SJ. Posterior transdiscal three-column shortening in the surgical treatment of vertebral discitis/osteomyelitis with collapse. Spine (Phila Pa 1976). 2010. 35: 1316-22
30. Herron LD, Kissel P, Smilovitz D. Treatment of coccidioidal spinal infection: experience in 16 cases. Journal of spinal disorders. 1997. 10: 215-22
31. . Increase in reported coccidioidomycosis--United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013. 62: 217-21
32. Jackson FE, Kent D, Clare F. Quadriplegia Caused by Involvement of Cervical Spine with Coccidioides Immitis. Symptomatic Cure after Operation and Amphotericin-B Treatment. J Neurosurg. 1964. 21: 512-15
33. Kakarla UK, Kalani MY, Sharma GK, Sonntag VK, Theodore N. Surgical management of coccidioidomycosis of the spine: clinical article. J Neurosurg Spine. 2011. 15: 441-46
34. Kirk KL, Kuklo TR. Back pain in a 22-year-old man. Clin Orthop Relat Res. 2003. 415: 319-28
35. Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013. 3: a010306-
36. Kushwaha VP, Shaw BA, Gerardi JA, Oppenheim WL. Musculoskeletal coccidioidomycosis. A review of 25 cases. Clin Orthop Relat Res. 1996. 332: 190-9
37. Lange T, Schulte TL, Bullmann V. Two recurrences of adjacent spondylodiscitis after initial surgical intervention with posterior stabilization, debridement, and reconstruction of the anterior column in a patient with spondylodiscitis: a case report. Spine (Phila Pa 1976). 2010. 35: E804-810
38. Laniado-Laborin R. Coccidioidomycosis and other endemic mycoses in Mexico. Rev Iberoam Micol. 2007. 24: 249-58
39. Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann N Y Acad Sci. 2007. 1111: 19-34
40. Lee DG, Park KB, Kang DH, Hwang SH, Jung JM, Han JW. A clinical analysis of surgical treatment for spontaneous spinal infection. J Korean Neurosurg Soc. 2007. 42: 317-325
41. Lewicky YM, Roberto RF, Curtin SL. The unique complications of coccidioidomycosis of the spine: a detailed time line of disease progression and suppression. Spine (Phila Pa 1976). 2004. 29: E435-441
42. Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003. 12: 606-12
43. Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. American journal of respiratory and critical care medicine. 2011. 183: 96-128
44. Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000. 25: 963-77
45. McGahan JP, Graves DS, Palmer PE. Coccidioidal spondylitis: usual and unusual radiographic manifestations. Radiology. 1980. 136: 5-9
46. Mertz LE, Blair JE. Coccidioidomycosis in rheumatology patients: incidence and potential risk factors. Ann N Y Acad Sci. 2007. 1111: 343-357
47. Olson EM, Duberg AC, Herron LD, Kissel P, Smilovitz D. Coccidioidal spondylitis: MR findings in 15 patients. AJR American journal of roentgenology. 1998. 171: 785-789
48. Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc. 2008. 83: 343-48
49. Prabhu RM, Bonnell M, Currier BL, Orenstein R. Successful treatment of disseminated nonmeningeal coccidioidomycosis with voriconazole. Clin Infect Dis. 2004. 39: e74-77
50. Reach P, Paugam A, Kahan A, Allanore Y, Wipff J. Coccidioidomycosis of the spine in an immunocompetent patient. Joint, bone, spine: Revue du rhumatisme. 2010. 77: 611-13
51. Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012. 272: 541-61
52. Sanchez CJ, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013. 13: 47-
53. Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci. 2007. 1111: 301-314
54. Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007. 45: 26-30
55. Schomacher M, Finger T, Koeppen D, Süss O, Vajkoczy P, Kroppenstedt S. Application of titanium and polyetheretherketone cages in the treatment of pyogenic spondylodiscitis. Clin Neurol Neurosurg. 2014. 127: 65-70
56. Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004. 22: 39-43
57. Stewart S, Barr S, Engiles J, Hickok NJ, Shapiro IM, Richardson DW. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: A proof-of-concept study. The Journal of bone and joint surgery American volume. 2012. 94: 1406-15
58. Szeyko LA, Taljanovic MS, Dzioba RB, Rapiejko JL, Adam RD. Vertebral coccidioidomycosis: Presentation and multidisciplinary management. Am J Med. 2012. 125: 304-14
59. Taljanovic MS, Adam RD. Musculoskeletal coccidioidomycosis. Semin Musculoskelet Radiol. 2011. 15: 511-526
60. Tan LA, Kasliwal MK, Nag S, O’Toole JE, Traynelis VC. Rapidly progressive quadriparesis heralding disseminated coccidioidomycosis in an immunocompetent patient. J Clin Neurosci. 2013. p.
61. Thrush A, Enzmann D. MR imaging of infectious spondylitis. AJNR Am J Neuroradiol. 1990. 11: 1171-80
62. Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007. 357: 654-63
63. Valdivia L, Nix D, Wright M, Lindberg E, Fagan T, Lieberman D. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerging infectious diseases. 2006. 12: 958-62
64. Wesselius LJ, Brooks RJ, Gall EP. Vertebral coccidioidomycosis presenting as Pott's disease. JAMA: The journal of the American Medical Association. 1977. 238: 1397-98
65. Winter WG, Larson RK, Zettas JP, Libke R. Coccidioidal spondylitis. The Journal of bone and joint surgery American volume. 1978. 60: 240-44
66. Wrobel CJ, Chappell ET, Taylor W. Clinical presentation, radiological findings, and treatment results of coccidioidomycosis involving the spine: report on 23 cases. J Neurosurg. 2001. 95: 33-39
67. Zeppa MA, Laorr A, Greenspan A, McGahan JP, Steinbach LS. Skeletal coccidioidomycosis: Imaging findings in 19 patients. Skeletal radiology. 1996. 25: 337-343