- Department of Neurosurgery, Nara Medical University, Kashihara, Japan
- Ohnishi Neurological Center, Akashi, Japan
Correspondence Address:
Yasushi Motoyama
Department of Neurosurgery, Nara Medical University, Kashihara, Japan
DOI:10.4103/2152-7806.174602
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Motoyama Y, Tanaka Y, Gurung P, Nakagawa I, Park Y, Nakase H. A simple bracing technique to correct kinking of arterial branches to avoid ischemic sequelae during neurovascular surgery. Surg Neurol Int 20-Jan-2016;7:8
How to cite this URL: Motoyama Y, Tanaka Y, Gurung P, Nakagawa I, Park Y, Nakase H. A simple bracing technique to correct kinking of arterial branches to avoid ischemic sequelae during neurovascular surgery. Surg Neurol Int 20-Jan-2016;7:8. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-simple-bracing-technique-to-correct-kinking-of-arterial-branches-to-avoid-ischemic-sequelae-during-neurovascular-surgery/
Abstract
Background:During microscopic procedures for neurovascular disease, we sometimes encounter kinking of arterial branches resulting in ischemic sequelae. A simple and useful technique that involves inserting a small, ball-like prosthesis made of oxidized cellulose or shredded Teflon with fibrin glue that corrects the arterial branch kinking and avoids subsequent compromise is reported.
Methods:Between January and December 2014, three patients developed arterial kinking during microscopic procedures, including two in the caudal loop of the posterior inferior cerebellar artery during microvascular decompression for glossopharyngeal neuralgia and one in a branch of the middle cerebral artery (MCA) during clipping for an unruptured MCA aneurysm. Blood flow insufficiency was confirmed by microvascular Doppler ultrasonography (MDU) and indocyanine green (ICG) videoangiography. The prosthesis, which was made of shredded Teflon in two cases and oxidized cellulose in one case, was inserted into the crotch of the kinked arteries to correct the kinking of the arteries and restore the proper vascular shape and normal blood flow.
Results:The small, ball-shaped prosthesis corrected the kinked arteries and maintained the proper shape, which was confirmed by ICG videoangiography and MDU during the operation and three-dimensional computerized tomography angiography postoperatively. Postoperatively, the patients did not manifest any ischemic sequelae related to the kinked arteries.
Conclusion:The insertion of prostheses with fibrin glue into the crotch of a kinked artery for repair is considered a simple and useful method for correcting a kinked artery that avoids ischemic sequelae.
Keywords: Cerebral aneurysm, clipping, glossopharyngeal neuralgia, kinking, microvascular decompression, simple bracing technique
INTRODUCTION
During clip application to middle cerebral artery (MCA) aneurysms, we occasionally encounter stenosis of a peripheral arterial branch resulting in ischemic sequelae caused by torsion or twisting of the branches of the MCA due to inappropriate clip application. To avoid such situations, some approaches, such as combination clip technique[
MATERIALS AND METHODS
Operative technique
Before surgery, the surgeon prepared various sizes of oxidized cellulose or shredded Teflon to use as the prosthesis for MVD or as material for hemostasis during the microscopic procedure in cases with neurovascular disease. Fibrin glue was also provided for such operations not only to seal the defect of the dura mater after microvascular surgery but also to use as an agent to enhance the effect on hemostasis with matrix such as oxidized cellulose or polyglycolic acid and holding the prosthesis in place to keep the offending vessel away from the root exit zone (REZ). When kinking of the arterial branch was observed during a microsurgical procedure, the small, ball-like prosthesis, made of shredded Teflon or oxidized cellulose soaked with thrombin solution, was applied to the crotch of the kinking site to correct the deformation of the artery and normalize the flow of the artery. Then, the fibrinogen solution of the fibrin glue was added to the ball to fix it to the kinked site to maintain the corrected shape and the normal flow of the artery. By placing the ball just near the crotch, it acted as a bolster held in place by the fibrin glue. During the microscopic procedure, microvascular Doppler ultrasonography (MDU) and indocyanine green (ICG) videoangiography were useful not only for identification of the kinked arterial branches, but also for verification of the correction of the kinked arteries and the patency of the arterial branches. The use of neurophysiological monitoring, such as motor-evoked potentials and sensory-evoked potentials, might also be able to help verify patency of the parent artery if the kinked artery contributed to the functional region. When three-dimensional computerized tomography angiography (3DCTA) was undertaken, the kinked artery could be confirmed to be corrected after surgery.
RESULTS
During the microscopic procedure, there was arterial branch kinking in three patients, including two with GPN and in one with an MCA aneurysm that was thought to be caused by thorough dissection of the arachnoid membrane and trabeculae. Correction of the kinked vessels was accomplished by insertion of a small, ball-like prosthesis into the crotch of the kinked artery. The balls were made of shredded Teflon fixed to the affected arteries by fibrin glue in two cases of MVD for GPN, and oxidized cellulose in the one case of MCA aneurysm surgery. Intraoperative ICG videoangiography and MDU were useful for detecting kinked arteries and confirming normalized flow of the corrected arteries. The patients’ postoperative courses were uneventful, and no other sequelae were observed in all patients. Even though the follow-up periods were only 2 years, no other complications, including infarction and recurrence, as well as granuloma formation, were observed.
CASE DESCRIPTION
Case 1
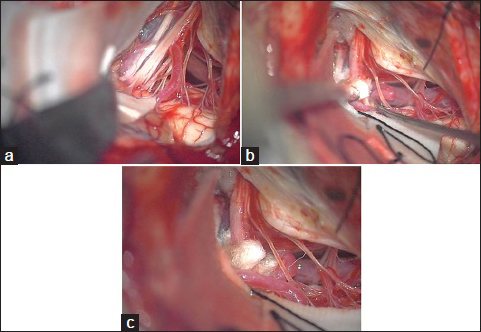
A 74-year-old man, presenting with a 4-year history of severe left throat pain while swallowing, was diagnosed with GPN, which was refractory to medical treatment. Because magnetic resonance imaging demonstrated the offending vessel compressing the REZ of the affected glossopharyngeal nerve, the patient underwent MVD for the GPN. During operation via a transcondylar fossa approach, the posterior inferior cerebellar artery (PICA) was confirmed to compress the origin of the glossopharyngeal nerve [
Figure 1
(a) Intraoperative microscopic view demonstrates that the posterior inferior cerebellar artery (white arrow) is compressing the glossopharyngeal nerve (black arrow), (red arrow: Vagal nerve). (b) After decompression, the caudal loop of the posterior inferior cerebellar artery is kinked (white arrow). (c) The inserted ball of shredded-Teflon soaked with the thrombin solution of the fibrin glue kit is held in place by the fibrinogen solution spray to correct the kinked branch of the posterior inferior cerebellar artery
After the procedure had been accomplished, the peripheral part of the PICA that was dissected for transposition to the REZ was observed to be kinked in the caudal side of the lower cranial nerves [
Case 2
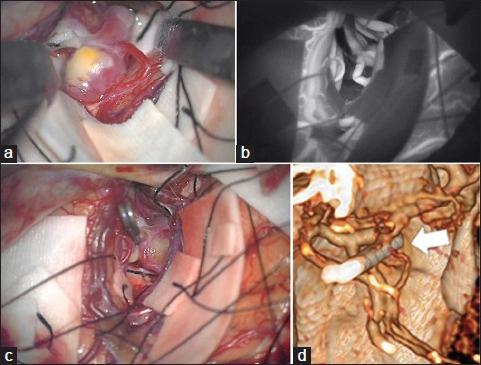
A 65-year-old man having an incidental aneurysm of more than 7 mm in his left MCA underwent surgical intervention. The aneurysm was approached through dissection of the sylvian fissure via a standard pterional craniotomy. The entire circumference of the dome of the aneurysm was dissected thoroughly [
Figure 2
(a) Intraoperative photograph shows the aneurysm originating from the bifurcation of the middle cerebral artery. (b) Indocyanine green videoangiography after application of the clip to the neck of the aneurysm shows the kinking of the superior trunk of the middle cerebral artery. (c) Insertion of the prosthesis made of crumpled oxidized cellulose with fibrin glue corrects the kinked frontal branch of the middle cerebral artery. (d) Postoperative three-dimensional computerized tomography angiography shows complete clipping of the aneurysm and no evidence of stenosis or kinking of the superior trunk of the middle cerebral artery (white arrow)
DISCUSSION
In neurosurgery to create a sufficient corridor to the lesion or obtain mobility of the vasculature for maneuverability, a variety of methods are used, including removal of the bony structure and dissection of supporting tissues, such as ronguering the sphenoid wing or anterior clinoid process, and dissection of the carotid dural ring.[
Kinking means that the vessel is extremely bent and the vessel loses the ability to maintain its lumen, so that the shape of the cross-section of the vessel changes from round to flat and collapses, at which point the vessel would be wound sharply. As a result, the lumen of the vessel narrows severely. In the vasculature, kinking of the vessel leads to insufficiency of blood flow and ischemic sequelae in cases of arterial branches and congestion in veins.
We sometimes observe kinking in extracranial carotid and vertebral arteries as one of the findings of atherosclerotic changes, as well as coiling or tortuosity.[
Kinking of the vessel may cause ischemia in arterial branches and congestion in veins. This insufficiency of blood flow could lead to infarction or swelling of the brain parenchyma and then a large variety of symptoms, depending on the areas affected. The details of the ischemic complications associated with kinking of vessels during microsurgery have not been previously reported. However, empirically, surgeons realize the danger of sequelae associated with kinking. Practically, the kinking of only one arterial branch would rarely cause severe complications. However, if other adverse factors, such as sacrifice of a large vein, long duration of the microscopic procedure, or excessive compression of the brain parenchyma with a spatula, would be combined with ischemia due to kinking of an arterial branch, the likelihood of brain damage would increase. In such situations, intermittent release of the spatula, a retractorless procedure,[
Troubleshooting for kinking of a vessel has never been described in detail before, even though its importance has been reported. If a step of the procedure was an immediate cause of the kinking, retracing of that step would be primary. When clip application to the neck causes the branch to twist, leading to kinking, release of the clip would resolve the kinking. Basically, a method of clip application that would not cause kinking is important. However, there may be many situations, in which reapplication of the clip is difficult, including an aneurysm with premature rupture. Another explanation is that the additional dissection to untether the branch more distally may also lead to resolution of kinking, by allowing the vessel to assume a gentler curve.
The bracing technique presented here is so simple and less invasive that it is very useful and feasible for prevention of postoperative complications. The immediate feedback provided by the intraoperative flow measurement by MDU and the confirmation of restoration of the proper shape on ICG videoangiography after resolution of kinking are important adjuncts to the success of this strategy.
In these patients, Teflon and oxidized cellulose were used. For a small, ball-shaped prosthesis inserted in the crotch of the kinked site, an appropriately sized material that can be easily shaped is needed. The prosthesis should also stay in position, and for this, an adherent agent as fibrin glue is necessary. Furthermore, the material should be able to maintain its size and shape for a period. When selecting the material, a sponge or other artificial material could be considered. However, side effects, such as a foreign body reaction, must be taken into account, so that alternatives such as an autograft of fascia, fat, and muscle have been used. Recently, granulation associated with Teflon implanted during surgery has been reported. Even though it is a rare complication, we must take such disadvantages into consideration and ensure long-term follow-up when selecting the materials for prostheses.
CONCLUSION
Thorough dissection of the arachnoid membrane and trabeculae can lead to kinking of arteries during microscopic procedures for neurovascular disease. Correction of kinked arteries using insertion of prosthesis is considered a simple and useful method for avoiding ischemic sequelae in neurovascular surgery. We should pay attention to the occurrence of kinked arteries that can cause ischemic sequelae during aneurysmal surgery and MVD that requires wide dissection of arachnoid membranes. ICG videoangiography and MDU are useful for the detection of kinking and confirmation of repair.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985. 62: 667-72
2. Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: Technical report. Neurosurgery. 1993. 33: 244-50
3. Ishikawa T, Nakayama N, Moroi J, Kobayashi N, Kawai H, Muto T. Concept of ideal closure line for clipping of middle cerebral artery aneurysms – Technical note. Neurol Med Chir (Tokyo). 2009. 49: 273-7
4. Katsuno M, Tanikawa R, Izumi N, Hashimoto M. A modified anterior temporal approach for low-position aneurysms of the upper basilar complex. Surg Neurol Int. 2015. 6: 10-
5. Kazumata K, Kamiyama H, Ishikawa T, Takizawa K, Maeda T, Makino K. Operative anatomy and classification of the sylvian veins for the distal transsylvian approach. Neurol Med Chir (Tokyo). 2003. 43: 427-33
6. Koizumi H, Fukamachi A, Nukui H. Postoperative subdural fluid collections in neurosurgery. Surg Neurol. 1987. 27: 147-53
7. Kondo A, Ishikawa J, Yamasaki Y, Konishi T. Microvascular decompression of cranial nerves, particularly of the 7 th cranial nerve. Neurol Med Chir (Tokyo). 1980. 20: 739-51
8. Mumoli N, Cei M. Asymptomatic carotid kinking. Circ J. 2008. 72: 682-3
9. Spetzler RF, Sanai N. The quiet revolution: Retractorless surgery for complex vascular and skull base lesions. J Neurosurg. 2012. 116: 291-300
10. Weibel J, Fields WS. Tortuosity, coiling, and kinking of the internal carotid artery. I. Etiology and radiographic anatomy. Neurology. 1965. 15: 7-18
11. Zhu W, Liu P, Tian Y, Gu Y, Xu B, Chen L. Complex middle cerebral artery aneurysms: A new classification based on the angioarchitecture and surgical strategies. Acta Neurochir (Wien). 2013. 155: 1481-91