- Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
- Department of Neurosurgery, Copenhagen University Hospital, Copenhagen, Denmark
- Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden
- Department of Medical Physics, Karolinska University Hospital, Stockholm, Sweden
Correspondence Address:
Jiri Bartek Jr.
Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
DOI:10.4103/2152-7806.176138
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sinclair G, Jr. JB, Martin H, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int 10-Feb-2016;7:
How to cite this URL: Sinclair G, Jr. JB, Martin H, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int 10-Feb-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/adaptive-hypofractionated-gamma-knife-radiosurgery-for-a-large-brainstem-metastasis-2/
Abstract
Background:To demonstrate how adaptive hypofractionated radiosurgery by gamma knife (GK) can be successfully utilized to treat a large brainstem metastasis - a novel approach to a challenging clinical situation.
Case Description:A 42-year-old woman, diagnosed with metastatic nonsmall cell lung cancer in July 2011, initially treated with chemotherapy and tyrosine kinase inhibitors, developed multiple brain metastases March 2013, with subsequent whole brain radiotherapy, after which a magnetic resonance imaging (MRI) showed a significant volume regression of all brain metastases. A follow-up MRI in October 2013 revealed a growing brainstem lesion of 26 mm. Linear accelerator-based radiotherapy and microsurgery were judged contraindicated, why the decision was made to treat the patient with three separate radiosurgical sessions during the course of 1 week, with an 18% tumor volume reduction demonstrated after the last treatment. Follow-up MRI 2.5 months after her radiosurgical treatment showed a tumor volume reduction of 67% compared to the 1st day of treatment. Later on, the patient developed a radiation-induced perilesional edema although without major clinical implications. An MRI at 12 months and 18-fluoro-deoxyglucose positron emission tomography of the brain at 13 months showed decreased edema with no signs of tumor recurrence. Despite disease progression during the last months of her life, the patient's condition remained overall acceptable.
Conclusion:GK-based stereotactic adaptive hypofractionation proved to be effective to achieve tumor control while limiting local adverse reactions. This surgical modality should be considered when managing larger brain lesions in critical areas.
Keywords: Biological equivalent dose, brainstem metastasis, Karnofsky scale, nonsmall cell lung cancer, peripheral/prescription dose, recursive partitioning analysis, stereotactic adaptive hypofractionated radiosurgery
INTRODUCTION
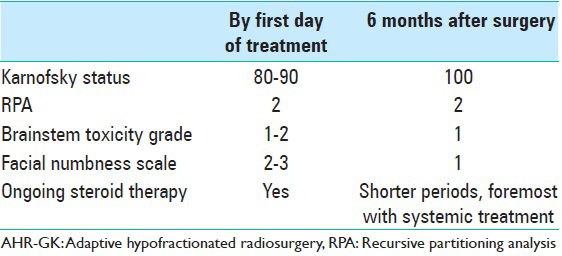
We present a case of a previously overall healthy 42-year-old female nonsmoker diagnosed with nonsmall cell lung cancer (NSCLC) in July 2011. Primary tumor screening proved liver and mediastinal lymph node involvement as well as the presence of a solitary L3 bone metastasis (T4N3M1a). The patient initially benefited from single tyrosine kinase inhibitor (TKI) therapy from July 2011 to March 2012 followed by combined chemotherapy and TKI treatment from April 2012 to December 2012, achieving next to complete regression of lung, liver, and nodal metastases. The lumbar metastasis remained stationary. In January 2013, the patient started TKI-maintenance therapy and underwent stereotactic body radiotherapy in order to treat a remaining lung lesion in the left lower lobe. In late March 2013, the patient developed onset of headaches, nausea, balance issues, intermittent perioral numbness, and fatigue. A computed tomography (CT)-scan and a magnetic resonance imaging (MRI) revealed at least 20 small cerebral lesions as well as a 20 mm brainstem lesion located in the pons. The patient started oral steroid therapy and underwent whole brain radiotherapy (WBRT) in April 2013 with 4 Gy daily fractions over 5 consecutive days (4 Gy × 5). An MRI performed 3 months after WBRT (July 2013) demonstrated next to complete regression of all small lesions; the pontine metastasis displayed a significant volume reduction of about 20% (20–16 mm). By this particular time, the patient's clinical status had also improved, only exhibiting symptoms of mild fatigue. A second follow-up MRI was performed in October 2013. The images revealed a striking tumor volume increase of the pontine lesion (16 mm in July to 26 mm in October 2013) as well as significant amount of perifocal edema, extending into the left side of the midbrain and cerebellar peduncles. By this time, the patient was experiencing mild left-sided second branch trigeminal nerve territory numbness (BNI-FN-Scale 2) as well as fatigue (Karnofsky scale 90). Further, extracranial radiological screening revealed limited thoracic metastatic activity (left lower lobe); hence, deductively recursive partitioning analysis (RPA)-class 2 [
RADIOSURGICAL ACCOUNT
The tumor had proven to be sensitive to prior WBRT and systemic treatment, and since microsurgery and further LINAC-based radiotherapy were earlier contraindicated, we resolved to treat the patient by means of AHR-GK. AHR-GK is an image-guided neurosurgical procedure aiming to dynamically treat larger brainstem lesions by means of nonhomogenously adjusted radiation doses within tumor boundaries in relation to ongoing tumor volume reduction through the course of radiotherapy. Sparing of healthy tissue while modifying dose delivery remains an important aspect of the treatment. In order to adapt radiation doses to expected morphological changes during the course of radiosurgery, a regimen was designed to deliver a total of three radiation treatments during the course of 6 days. Each fraction was to be delivered every 48 h. Optimal cranium fixation was achieved using the Leksell® stereotactic frame preceding each treatment. In order to attain capital dynamic target definition, stereotactic MRI-based treatment plans were performed prior each treatment. No additional plan margins were required other than the gross tumor volume (GTV = GTV, planning target volume not required because of frame-based stereotactic conditions). A renewed clinical examination preceding treatment initiation (treatment number 1), showed some exacerbation of the left side facial numbness (BNI-FN-Scale 2–3), fluctuating body temperature, and tiredness (Karnofsky scale 80–90). Brainstem toxicity (By CTEP-US Cancer Institute standards) at first treatment was graded as 1–2 [Tables
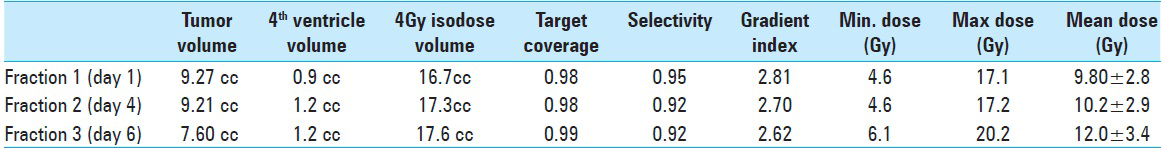
A strategic pretreatment planning was set upon previous MRI, past institutional experience, and vital patient data (tumor histopathological traits, tumor size, tumor localization, anatomical landmarks, previous response to intra- and extra-cranial radiation, radiobiological conditions due to prior WBRT and current knowledge on brainstem toxicity as well as clinical status by the time of radiosurgery). Institutional experience and world medical data show that larger metastases are likely to respond to physical dose regimens of 6–7 Gy given in 5 daily fractions (6–7 Gy × 5). An isoeffective regimen of three fractions was conceived using conventional linear-quadratic-model/biological equivalent dose estimates. Since the patient had previously been subjected to WBRT (physical dose regimen of 4 Gy's in five daily fractions = 4 Gy × 5), we had to apply a lower prescription dose to the margin in order to maximize healthy brainstem tissue sparing. Based on isoeffective estimations corresponding to prior WBRT (4 Gy × 5) and brainstem axis radiation tolerance data, we decided to set the prescription (marginal) dose between 6 and 7 Gy. To achieve best (maximal) dose distribution to target, the peripheral prescription dose was set at the 35% line. As a way to “monitor” dose dissipation to adjacent healthy tissues while increasing the prescription dose, treatment volumes to the 4 Gy isodose were to be kept as low and comparable as possible [
Before treatment start, the patient was thoroughly informed of the upcoming procedure, strategies, goals, and potential local adverse reactions. The patient was positive to treatment and started oral corticosteroid therapy (betamethasone) due to extensive preexisting edema.
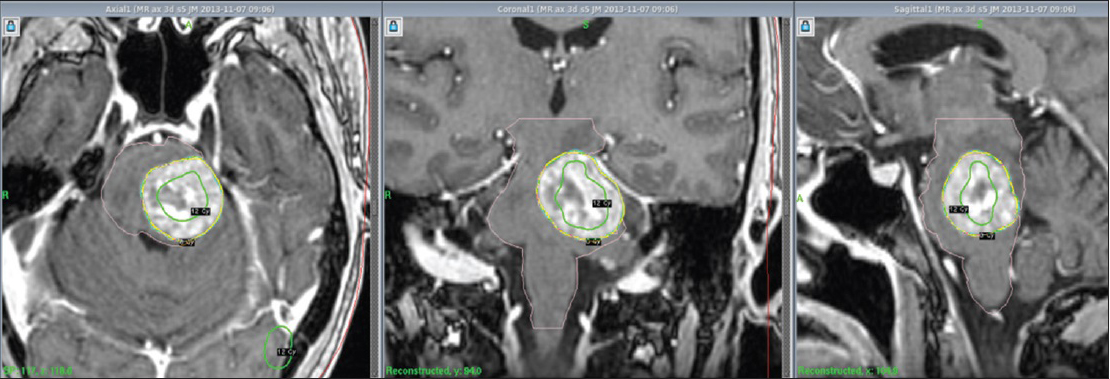
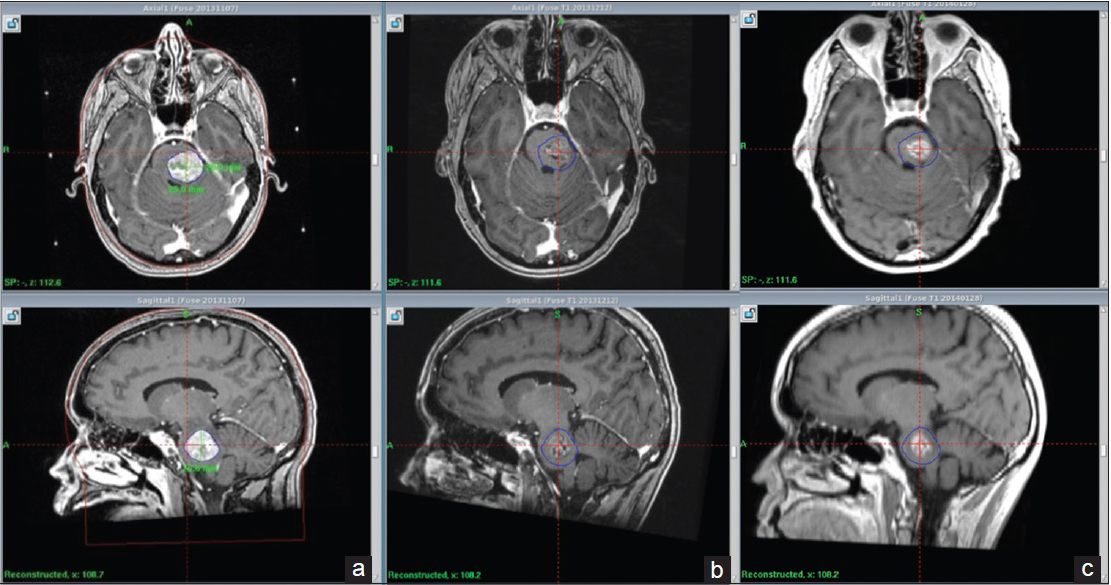
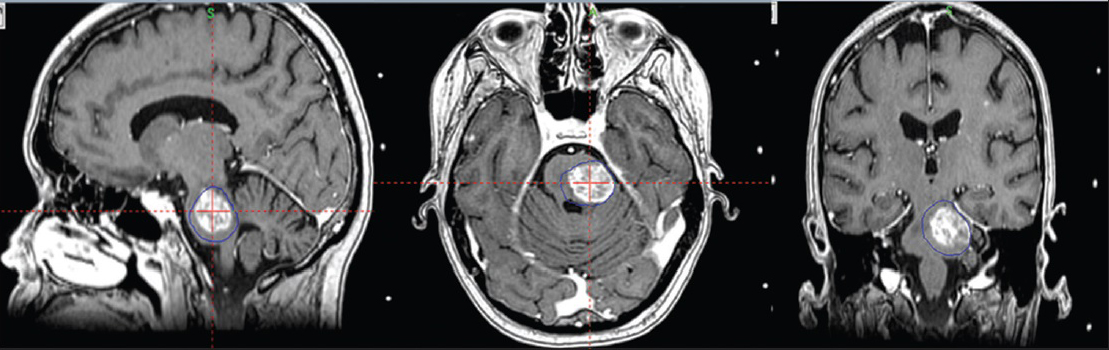
By the 1st day of treatment, the stereotactic MRI showed a slight volume progression of the brainstem metastasis; Leksell GammaPlan® volumetric measurements estimated a target volume of 9, 27 cc. Fluid-attenuated inversion recovery and other T2-weighted-sequences exhibited a considerable amount of edema within the left pontine boundaries, tectum, and left cerebellar peduncle. A renewed assessment based on all present factors confirmed a peripheral dose of 6 Gy to the 35% isodose for the first treatment. “Hot spots” were circumscribed to the 12 Gy isodose covering areas with the strongest contrast enhancement [
Because of logistics, the second fraction was delivered 3 days later (day 4); the second stereotactic MRI displayed no significant tumor reduction. There was somewhat less edema. The patient was still on steroids but showed no clinical improvement. We proceeded to deliver a second fraction of 6 Gy to the 35% isodose line.
The third and final treatment was given 2 days later (day 6). Its correspondent stereotactic MRI revealed an 18% volume decrement compared to the previous stereotactic MRI [
The patient remained on cortisone throughout her treatments. Steroids were rapidly de-escalated after surgery completion. Frame application 3 times a week was well tolerated by the patient.
OUTCOME
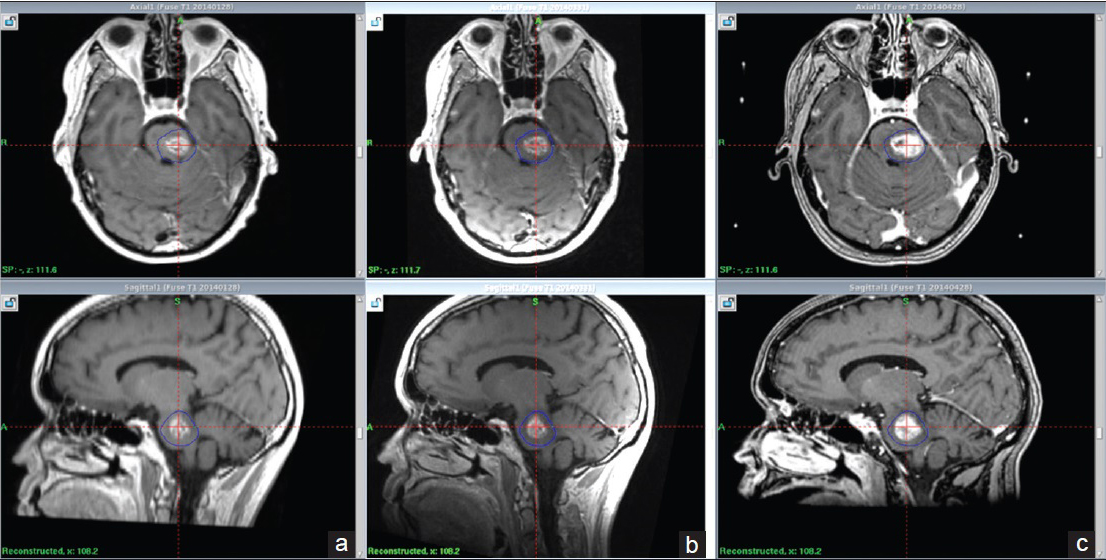
Follow-up MRIs at one and 2.5 months after radiosurgery (follow-up MRI numbers 1 and 2) showed a tumor volume abatement of about 67% during the course of 8 weeks [
Follow-up MRI at 4.5 and 5.5 months (follow-up MRI 4 and 5) showed the progression of tumor size, with edema extending into the midbrain, pons, and left cerebellar peduncle. A local radiation adverse effect was initially suspected and indeed expected [Figure
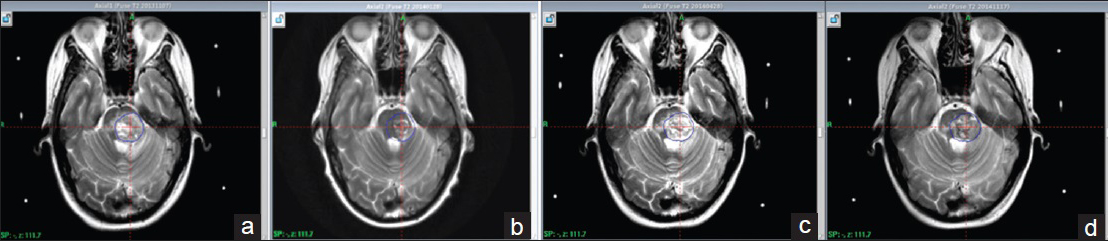
Figure 4
T2 weighted series. Pictorial summary concerning follow up of edema from first day of treatment. Substantial amount of underlying edema at the first day of treatment (a) resolving 2.5 months after treatment (b). MRI at 5.5 months show new development of edema indication a radiation induced local reaction (c). MRI at 12 months displays reduction of perifocal edema suggesting the adverse reaction is once again resolving (d)
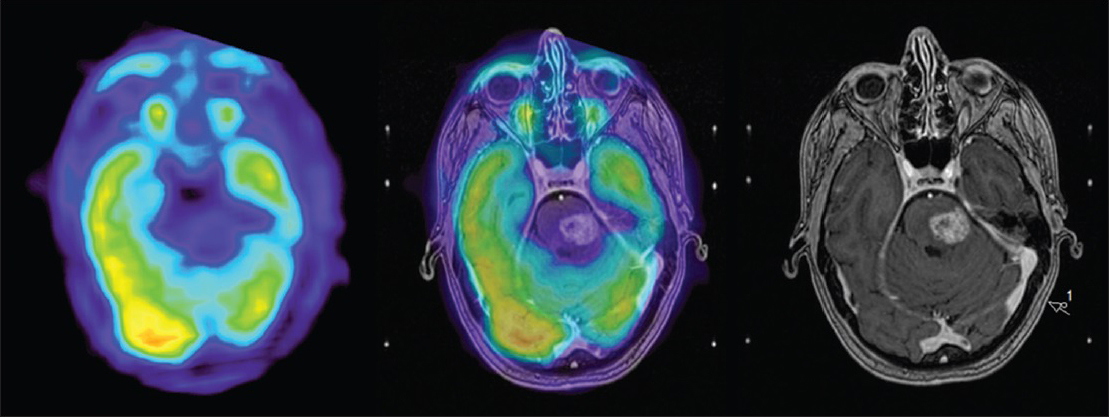
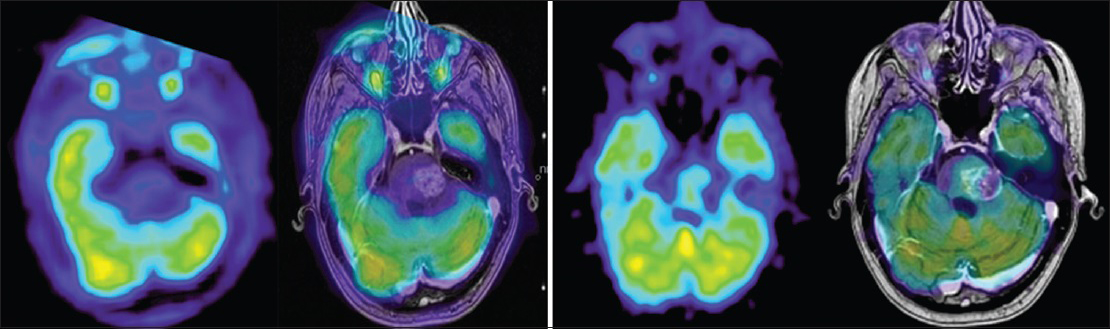
An FDG-PET at 5.5 months after treatment was thus performed (follow-up FDG-PET number 1) showing limited local uptake, confirming our suspicions of a local radiation adverse reaction [
Follow-up MRI 8, 9, 10 months after radiosurgery (follow-up MRI numbers 5, 6, 7) showed stable conditions with no further brainstem tumor volume progression or supplementary edema. A new MRI taken at 12 months described a light depletion of local contrast enhancement, tumor volume, and adjacent edema [
Figure 7
FDG-PET and co-registered contrast enhanced T1 weighted image at 12 months (right group): Tumor to contralateral normal frontal gray matter ratio still <1 with no signs of viable tumor compared to FDG-PET from whole body PET (delayed phase) and co-registered contrast enhanced T1 weighted image at 5.5 months (left group)
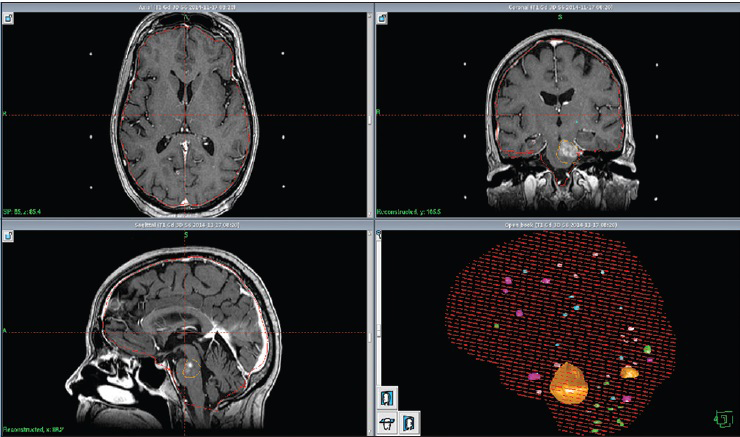
The patient suffered a major primary tumor progression in June 2014. Because of low tolerance/growing resistance to systemic treatment, the patient developed further distant metastatic disease including new brain metastases. Between April and November 2014, a total of 40 new metastases were successively treated by single fraction [
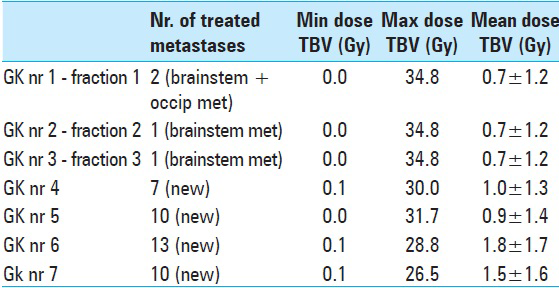
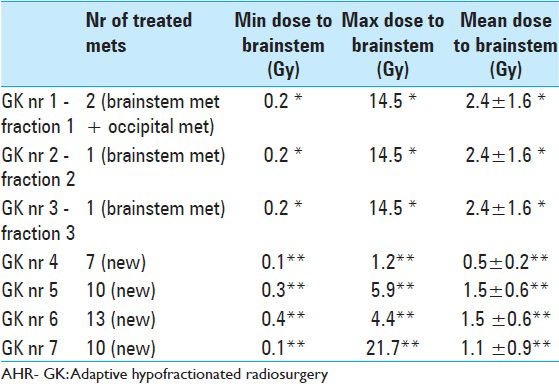
Table 9
LGP (Leksell GammaPlan®) based brainstem dose estimations corresponding to each Gamma Knife session (GK nr) between November 2013 and November 2014. Minimum (Min), maximum (Max) and mean radiation dose values are given in Gray Unit (Gy). Estimations corresponding to AHR- GK treatments 1 to 3 are based on a ‘normal’ brainstem tissue volume (pons metastasis not included)* Dose estimations corresponding to treatments 4,5,6,7 are based on a total brainstem Volume comprehending healthy brainstem tissues and the previously treated pons metastasis (both components were regarded by that time as same organ at risk)**
Unfortunately, a CT-scan performed by late November 2014 showed additional thoracic and skeletal disease despite different anti-tumoral regimens. By that time, main clinical issues were described as fatigue and skin rash mainly due to systemic therapy (Karnofsky 70, RPA 3). No major neurological deficit or cognitive impairment was described at this stage. The brainstem metastasis remained inactive. Failing systemic treatment, the patient died in early February 2015.
DISCUSSION
The brainstem is a highly eloquent organ with special architectural traits and complex neurophysiological functions. Although brainstem metastases account for only 5% of all intracranial metastases, these cases are usually difficult to manage because of its critical localization, often leading to significant neurological impairment. At present, there is no consensus on the optimal approach to treat these malignant neoplasms, especially in the case of local recurrence after radiation treatments such as WBRT. The role of radiosurgery in the treatment of smaller brainstem metastases has been well described in the last few years[
World medical literature[
Early studies promptly identified the relationship between prescription dose and irradiated brainstem volume in terms of toxicity. In the present day, the entire brainstem axis is known to have a radiation tolerance of up to 54 Gy using conventional fractionation of 1.8–2.0 Gy/day. Smaller volumes (up to 10 cc) may be treated to maximum doses of up to 59 Gy for conventional fractionation.[
When considering the average brainstem volume in the adult,[
Our institutional experience in single fraction treatments supports the importance of dose-volume relations. the risk of local adverse events following a single treatment of intrinsic or adjacent brainstem tumors remains acceptable as long as its correspondent 10 Gy volume (to the brainstem) is kept within the 3 cc's circumscription. Furthermore, in day-to-day clinical practice, we stand somewhat close to Radiation Therapy Oncology Group (ROTG) 90–05 dose-volume recommendations of 24, 18, and 15 Gy for metastatic lesions of diameter <20, 21–30, and 31–40 mm, respectively.[
Extrapolating all of the above information onto a practical hypofractionated regimen dealing with local tumor control and restrained local toxicity is a matter of much debate nowadays, particularly facing inhomogeneous dose distributions. It is still highly dependent on the radiosurgical team's consensus on available mathematical/radiobiological models and subsequent isoeffective dose estimations/conversions.[
In our case, major challenges proved to be previous WBRT as well as underlying (and somehow cortisone-resistant) edema, theoretically increasing the risk for local toxicity, hence limiting radiation delivery. Considering the above, it was of outmost importance to prescribe a peripheral dosage able to “cumulate” as much radiation as possible within tumor bed boundaries (especially to contrast enhancing areas) while keeping radiation dissipation outside target as low and homogeneous as possible [
Moreover, we believe RPA classes (among other prognostic classifications not mentioned in this paper) play a major predictive roll in the outcome of brain radiosurgery, especially in eloquent regions because of the likelihood of better response, we recommend the above technique to be limited to patients with RPA–classes 1 and 2.[
Finally, much can be debated on the role the tumor's own radio-sensitivity and prior given WBRT played in the treatment's positive outcome was AHR-GK a “boost” procedure as such? Could comparable results, in similar conditions, be achieved when facing radio-resistant histologies? The latter will be the main subject of another paper.
CONCLUSION
In terms of local tumor control and radiation tolerance, GK-based AHR-GK could be seen as an alternative neurosurgical approach in the management of large brain metastases in critical areas. Further studies into brainstem toxicity are required to improve the radio-therapeutics involved in these cases. Hypofractionation regimens should be individually assessed by means of imaging, relevant clinical information, dose-volume data, and the surgical team's commodity to work with available mathematical/radiobiological models.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Roberto Martνnez Αlvarez and Dr. Nuria E. Martνnez Moreno (Department of Functional Neurosurgery, Hospital Ruber Internacional, Madrid, Spain) for all helpful comments and suggestions regarding this paper.
References
1. Andisheh B, Edgren M, Belkic D, Mavroidis P, Brahme A, Lind BK. A comparative analysis of radiobiological models for cell surviving fractions at high doses. Technol Cancer Res Treat. 2013. 12: 183-92
2. Arrieta O, Villarreal-Garza C, Zamora J, Blake-Cerda M, de la Mata MD, Zavala DG. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011. 6: 166-
3. Beli I, Koukourakis G, Platoni K, Tolia M, Kelekis N, Kouvaris J. Hypofractionated radiotherapy in non small cell lung cancer: A review of the current literature. Rev Recent Clin Trials. 2010. 5: 103-11
4. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved?. Int J Radiat Oncol Biol Phys. 2014. 88: 254-62
5. Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012. 83: 303-9
6. Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001. 96: 191-7
7. Clark BG, Souhami L, Pla C, Al-Amro AS, Bahary JP, Villemure JG. The integral biologically effective dose to predict brain stem toxicity of hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1998. 40: 667-75
8. Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Grégoire V. Early reoxygenation in tumors after irradiation: Determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005. 63: 901-10
9. Dawe DE, Greenspoon JN, Ellis PM. Brain metastases in non-small-cell lung cancer. Clin Lung Cancer. 2014. 15: 249-57
10. Erbagci H, Keser M, Kervancioglu S, Kizilkan N. Estimation of the brain stem volume by stereological method on magnetic resonance imaging. Surg Radiol Anat. 2012. 34: 819-24
11. Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R. Hypofractionated stereotactic radiotherapy for brain metastases - results from three different dose concepts. Strahlenther Onkol. 2007. 183: 625-30
12. Faria SL. Role of radiotherapy in metastatic non-small cell lung cancer. Front Oncol. 2014. 4: 229-
13. Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010. 83: 554-68
14. Fuentes S, Delsanti C, Metellus P, Peragut JC, Grisoli F, Regis J. Brainstem metastases: Management using gamma knife radiosurgery. Neurosurgery. 2006. 58: 37-42
15. Ganz JC.editors. Gamma Knife Neurosurgery. Vienna: Springer; 2011. p.
16. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37: 745-51
17. Grossman R, Ram Z. Recursive partitioning analysis (RPA) classification predicts survival in patients with brain metastases from sarcoma. World Neurosurg. 2014. 82: 1291-4
18. Hatiboglu MA, Chang EL, Suki D, Sawaya R, Wildrick DM, Weinberg JS. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011. 69: 796-806
19. Hoban PW, Jones LC, Clark BG. Modeling late effects in hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1999. 43: 199-210
20. Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J. Five-fraction Cyberknife radiotherapy for large brain metastases in critical areas: Impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res. 2014. 55: 334-42
21. Inoue HK, Sato H, Suzuki Y, Saitoh J, Noda SE, Seto K. Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: A single-institutional prospective study. Radiat Oncol. 2014. 9: 231-
22. Kased N, Huang K, Nakamura JL, Sahgal A, Larson DA, McDermott MW. Gamma knife radiosurgery for brainstem metastases: The UCSF experience. J Neurooncol. 2008. 86: 195-205
23. Kawabe T, Yamamoto M, Sato Y, Barfod BE, Urakawa Y, Kasuya H. Gamma Knife surgery for patients with brainstem metastases. J Neurosurg. 2012. 117: S23-30
24. Kelly PJ, Lin YB, Yu AY, Ropper AE, Nguyen PL, Marcus KJ. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: The Dana-Farber/Brigham and Women's Cancer Center experience. J Neurooncol. 2011. 104: 553-7
25. Kilburn JM, Ellis TL, Lovato JF, Urbanic JJ, Bourland JD, Munley MT. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: Is there a volume threshold for toxicity?. J Neurooncol. 2014. 117: 167-74
26. Kissick M, Campos D, van der Kogel A, Kimple R. On the importance of prompt oxygen changes for hypofractionated radiation treatments. Phys Med Biol. 2013. 58: N279-85
27. Koyfman SA, Tendulkar RD, Chao ST, Vogelbaum MA, Barnett GH, Angelov L. Stereotactic radiosurgery for single brainstem metastases: The cleveland clinic experience. Int J Radiat Oncol Biol Phys. 2010. 78: 409-14
28. Kurtz G, Zadeh G, Gingras-Hill G, Millar BA, Laperriere NJ, Bernstein M. Salvage radiosurgery for brain metastases: Prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014. 88: 137-42
29. Lamm AF, Elaimy AL, Lamoreaux WT, Mackay AR, Fairbanks RK, Demakas JJ. A review of the clinical outcomes for patients diagnosed with brainstemmetastasis and treated with stereotactic radiosurgery. ISRN Surg 2013. 2013. p.
30. Leeman JE, Clump DA, Wegner RE, Heron DE, Burton SA, Mintz AH. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol. 2012. 7: 107-
31. Li Y, Xu D, Zhang Z, Zhang Y, Liu D, Liu X. Gamma Knife surgery for brainstem metastases. J Neurosurg. 2012. 117: S13-6
32. Likhacheva A, Pinnix CC, Parikh NR, Allen PK, McAleer MF, Chiu MS. Predictors of survival in contemporary practice after initial radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013. 85: 656-61
33. Lin G, Xu H, Huang C. Advances in treatment of brain metastases from primary non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2014. 17: 877-83
34. Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Bürk K. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex. 1999. 9: 712-21
35. Ma LH, Li G, Zhang HW, Wang ZY, Dang J, Zhang S. Hypofractionated stereotactic radiotherapy with or without whole-brain radiotherapy for patients with newly diagnosed brain metastases from non-small cell lung cancer. J Neurosurg. 2012. 117: S49-56
36. Mangel L, Skriba Z, Major T, Polgár C, Fodor J, Somogyi A. Modelling normal tissue isoeffect distribution in conformal radiotherapy of glioblastoma provides an alternative dose escalation pattern through hypofractionation without reducing the total dose. Acta Oncol. 2002. 41: 162-8
37. Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010. 76: S36-41
38. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004. 5: 429-41
39. Nieder C, Nestle U, Motaref B, Walter K, Niewald M, Schnabel K. Prognostic factors in brain metastases: Should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes?. Int J Radiat Oncol Biol Phys. 2000. 46: 297-302
40. Niranjan A. Conformity index for radiosurgery. Neurosurgery. 2010. 67: E521-
41. Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008. 70: 847-52
42. Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol. 2011. 32: 1885-92
43. Peterson HE, Larson EW, Fairbanks RK, MacKay AR, Lamoreaux WT, Call JA. Gamma knife treatment of brainstem metastases. Int J Mol Sci. 2014. 15: 9748-61
44. Prasanna A, Ahmed MM, Mohiuddin M, Coleman CN. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J Thorac Dis. 2014. 6: 287-302
45. Ricciardi S, de Marinis F. Multimodality management of non-small cell lung cancer patients with brain metastases. Curr Opin Oncol. 2010. 22: 86-93
46. Santacroce A, Kamp MA, Budach W, Hänggi D. Radiobiology of radiosurgery for the central nervous system. Biomed Res Int 2013. 2013. p.
47. Schlienger M, Nataf F, Huguet F, Pene F, Foulquier JN, Orthuon A. Hypofractionated stereotactic radiotherapy for brain metastases. Cancer Radiother. 2010. 14: 119-27
48. Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: Initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996. 34: 647-54
49. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000. 47: 291-8
50. Sheu T, Molkentine J, Transtrum MK, Buchholz TA, Withers HR, Thames HD. Use of the LQ model with large fraction sizes results in underestimation of isoeffect doses. Radiother Oncol. 2013. 109: 21-5
51. Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro Oncol. 2013. 15: 515-34
52. Vetlova ER, Golanov AV, Gorlachev GE, Dalechina AV, Pronin IN, Dolgushin MB. Stereotactic radiotherapy for cerebral metastases using CyberKnife. Zh Vopr Neirokhir Im N N Burdenko. 2012. 76: 37-45
53. Wang SX, Boethius J, Ericson K. FDG-PET on irradiated brain tumor: Ten years’ summary. Acta Radiol. 2006. 47: 85-90
54. Xue J, Goldman HW, Grimm J, LaCouture T, Chen Y, Hughes L. Dose-volume effects on brainstem dose tolerance in radiosurgery. J Neurosurg. 2012. 117: S189-96
55. Yen CP, Sheehan J, Patterson G, Steiner L. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006. 105: 213-9
56. Yomo S, Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. 2013. 113: 459-65
57. Yoo TW, Park ES, Kwon do H, Kim CJ. Gamma knife radiosurgery for brainstem metastasis. J Korean Neurosurg Soc. 2011. 50: 299-303
58. Zada G, Yu C, Pagnini PG, Khalessi AA, Zelman V, Apuzzo ML. Early decreased tumor volume following fractionated Gamma Knife Radiosurgery for metastatic melanoma and the role of “adaptive radiosurgery”: Case report. Neurosurgery. 2010. 67: E512-3
59. Debus J, Hug EB, Liebsch NJ, O’Farrel D, Finkelstein D, Efird J. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997. 39: 967-75