- Department of Neurology and Psychiatry, Division of Neurosurgery, Sapienza University of Rome, Rome, Italy
- Department of Neurosurgery, INM Neuromed, Pozzilli, Italy
- Department of Diagnostic and Therapeutic Neuroradiology, INM Neuromed, Pozzilli, Italy
- Department of Pediatric Neurology, UTHSC (University of Tennessee Health Science Center), Memphis, Tennessee, USA
- Department of Experimental Biomedicine and Clinical Neurosciences, Neurosurgical Clinic, University of Palermo, Palermo, Italy
Correspondence Address:
Alessandro Landi
Department of Neurology and Psychiatry, Division of Neurosurgery, Sapienza University of Rome, Rome, Italy
DOI:10.4103/2152-7806.191082
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Landi A, Innocenzi G, Grasso G, Meschini A, Fabbiano F, Castri P, Delfini R. Diagnostic potential of the diffusion tensor tractography with fractional anisotropy in the diagnosis and treatment of cervical spondylotic and posttraumatic myelopathy. Surg Neurol Int 22-Sep-2016;7:
How to cite this URL: Landi A, Innocenzi G, Grasso G, Meschini A, Fabbiano F, Castri P, Delfini R. Diagnostic potential of the diffusion tensor tractography with fractional anisotropy in the diagnosis and treatment of cervical spondylotic and posttraumatic myelopathy. Surg Neurol Int 22-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/diagnostic-potential-diffusion-tensor-tractography-fractional-anisotropy-diagnosis-treatment-cervical-spondylotic-posttraumatic-myelopathy/
Abstract
Background:Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI)-based methodology widely used for the evaluation of microstructural integrity of the central nervous system (CNS), particularly of brain white matter fibers and bundles.
Methods:The most common parameters evaluated in a DTI study are the fractional anisotropy (FA) and mean diffusivity (MD). Combining FA and MD analyses is commonly used in the evaluation of various types of brain pathologies, such as brain tumors, where a combined analysis allows an accurate tumor characterization.
Results:Recent studies have shown that FA and MD could be of value in non-oncologic spinal pathology. In this regard, it has been demonstrated that DTI can provide new insights into the diagnosis and prognosis of cervical spondylotic myelopathy and cervical spinal cord injury.
Conclusions:Further studies are needed to assess the role of DTI in such a new clinical scenario.
Keywords: Cervical myelopathy, diffusion tensor imaging, fiber tracking, fractional anisotropy, magnetic resonance imaging, spinal cord injury
INTRODUCTION
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI)-based methodology widely used for the evaluation of microstructural integrity of the central nervous system (CNS), particularly of brain white matter fibers and bundles.[
CERVICAL SPONDYLOTIC MYELOPATHY
CSM is characterized by a progressive degeneration of the cervical spinal motor unit caused by osteoarthritis or degenerative chronic compressive pathology; MRI T2 MR sequences are most commonly used to assess cord damage. Hyperintense cord signals on T2 sequences are utilized to document myelomalacia.[
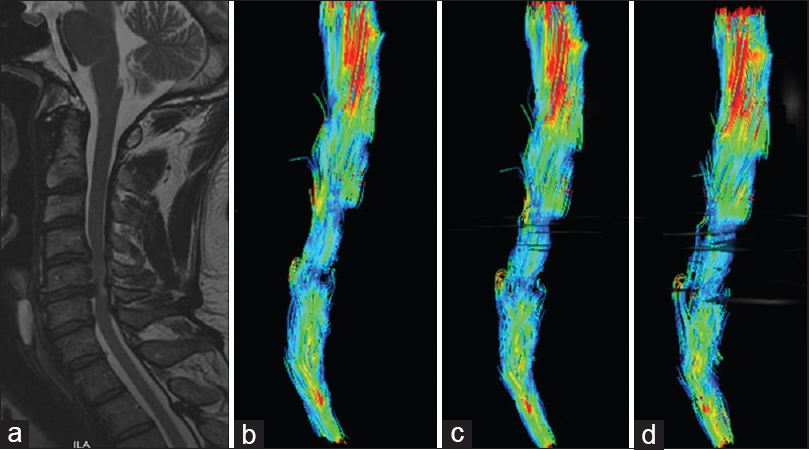
Figure 1
A 67-year-old man presented with a progressive paraparesis. Sagittal T2 weighted magnetic resonance images showed spinal cord compression with hyperintense cord signals at C5-C6 level (a). Diffusion tensor imaging showed decreasing in fractional anisotropy at C5-C6 level (b) but lower, at C4-C5 segment (c, d)
Postoperative utility of T2 magnetic resonance: Fractional anisotropy and mean diffusivity analyses
Recent studies have suggested that T2 MRI could not be utilized to successfully correlate the extent of neurological damage with outcomes for patients after surgery for CSM.[
TRAUMATIC SPINAL CORD INJURY
Little is known regarding the potential usefulness of FA and MD as diagnostic and prognostic tools in assessing traumatic spinal cord injury (SCI).[
Utility to demonstrate when and whether spinal surgery for spinal cord injury is indicated
Emergent surgical treatment (e.g., decompression) may also be indicated when SCI correlates with clinical symptoms and MRI T2 hyperintensities. For example, there may be a symptomatic patient with negative T2 MRI findings who only requires conservative treatment. Conversely, microstructural damage highlighted by MR-documented FA and MD as well as hyperintense signals on T2 MR studies may indicate that emergency surgical decompression is warranted.
The impact of FA and MD in the evaluation of the spinal trauma is challenging. Unfortunately, these instruments, as well as the software dedicated to the elaboration of specific sequences, are not yet widely available.
CONCLUSIONS
The development of imaging techniques, such as novel MRI methods, including FA and MD sequences, provide for improved treatment options, and are especially useful in the field of complex and delicate pathologies of the spinal cord. Additional research is required to confirm the preliminary data, and multicenter prospective randomized trials will allow the clinical validation of these methods and enable their subsequent clinical application.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baehring JM, Fulbright RK. Diffusion-weighted MRI in neuro-oncology. CNS Oncol. 2012. 1: 155-67
2. Cui JL, Wen CY, Hu Y, Li TH, Luk KD. Entropy-based analysis for diffusion anisotropy mapping of healthy and myelopathic spinal cord. Neuroimage. 2011. 54: 2125-31
3. Ellingson BM, Salamon N, Hardy AJ, Holly LT. Prediction of Neurological Impairment in Cervical Spondylotic Myelopathy using a Combination of Diffusion MRI and Proton MR Spectroscopy. PLoS One. 2015. 10: e0139451-
4. Guan X, Fan G, Wu X, Gu G, Gu X, Zhang H. Diffusion tensor imaging studies of cervical spondylotic myelopathy: A systemic review and meta-analysis. PLoS One. 2015. 10: e0117707-
5. Landi A, Palmarini V, D’Elia A, Marotta N, Salvati M, Santoro A. Magnetic resonance diffusion tensor imaging and fiber-tracking diffusion tensor tractography in the management of spinal astrocytomas. World J Clin Cases. 2016. 4: 1-4
6. Lin E, Long H, Li G, Lei W. Does diffusion tensor data reflect pathological changes in the spinal cord with chronic injury. Neural Regen Res. 2013. 8: 3382-90
7. Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: Evaluation of the “tract of interest” by diffusion tensor tractography. AJNR Am J Neuroradiol. 2006. 27: 1040-5
8. Wang F, Sun T, Li XG, Liu NJ. Diffusion tensor tractography of the temporal stem on the inferior limiting sulcus. J Neurosurg. 2008. 108: 775-781
9. Wang SQ, Li X, Cui JL, Li HX, Luk KD, Hu Y. Prediction of myelopathic level in cervical spondylotic myelopathy using diffusion tensor imaging. J Magn Reson Imaging. 2015. 41: 1682-8
10. Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He J. Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. Spine. 2010. 35: E396-9
11. Zhao C, Rao JS, Pei XJ, Lei JF, Wang ZJ, Yang ZY. Longitudinal study on diffusion tensor imaging and diffusion tensor tractography following spinal cord contusion injury in rats. Neuroradiology. 2016. 58: 607-14