- Department of Neurosurgery, China Medical University Hospital, Taichung, Taiwan
- Department of Radiology, China Medical University Hospital, Taichung, Taiwan
Correspondence Address:
Han-Chung Lee
Department of Neurosurgery, China Medical University Hospital, Taichung, Taiwan
DOI:10.4103/2152-7806.157794
Copyright: © 2015 Hsiao H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Hsiao I, Lee H, Yen P, Cho D. Embolization followed by surgery for treatment of perimedullary arteriovenous fistula causing acute myelopathy. Surg Neurol Int 28-May-2015;6:
How to cite this URL: Hsiao I, Lee H, Yen P, Cho D. Embolization followed by surgery for treatment of perimedullary arteriovenous fistula causing acute myelopathy. Surg Neurol Int 28-May-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/embolization-followed-surgery-treatment-perimedullary/
Abstract
Background:Perimedullary arteriovenous fistula (AVF) is rare. There are three subtypes, and the treatment strategies for each are different. Subtype B (multiple fistulas) can be treated by either embolization or surgery. On the basis of a case from our treatment experience, we propose a method for achieving optimal outcome while minimizing nerve injury.
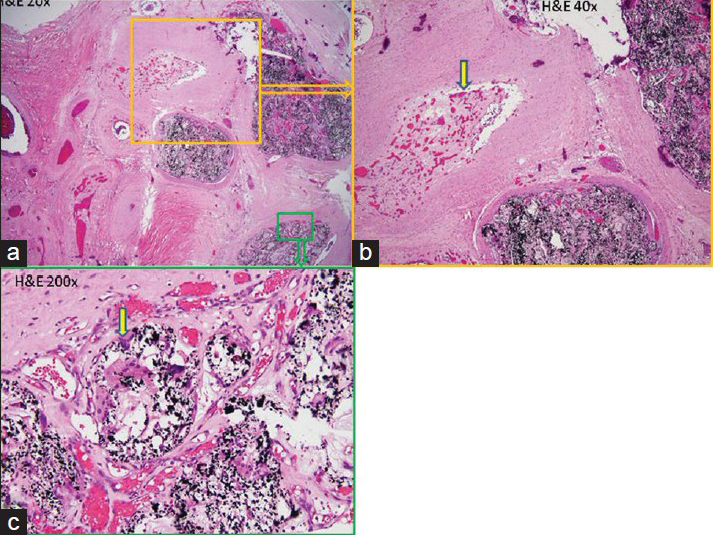
Case Description:A 51-year-old female was admitted to our hospital with acute myelopathy caused by a perimedullary AVF. Initially, we treated her by embolization using the chemical agent Onyx. Her symptoms improved immediately but gradually returned beginning 1 week later. Two months later, the symptoms had returned to pretreatment status, so we removed the fistulas surgically. Severe adhesions between nerve and occult venous varices were noted during the operation. Afterward, the patient's symptoms improved significantly. Histopathological sections showed an inflammatory reaction around the varices.
Conclusions:We initially considered several possible reasons for the return of symptoms: (a) Hypoperfusion of the spinal cord; (b) mass effect of the occult vein varices; (c) residual AVF or vascular remodeling resulting in recurrent cord hypertension; (d) Onyx-induced perivascular inflammation resulting in nerves adhering to each other and to occult venous varices. Clinical, surgical, and pathological findings ruled out the first three, leaving Onyx-induced perivascular inflammation as the probable reason. Given our treatment experience and the pros and cons of the two methods, we propose that initial embolization followed by surgery after 5 days to remove occult venous varices is the ideal strategy for treating perimedullary AVF of subtype B.
Keywords: Embolic material, Onyx, perimedullary arteriovenous fistula, spine
INTRODUCTION
Spinal vascular malformations are rare and can generally be divided into four types:[
CASE DESCRIPTION
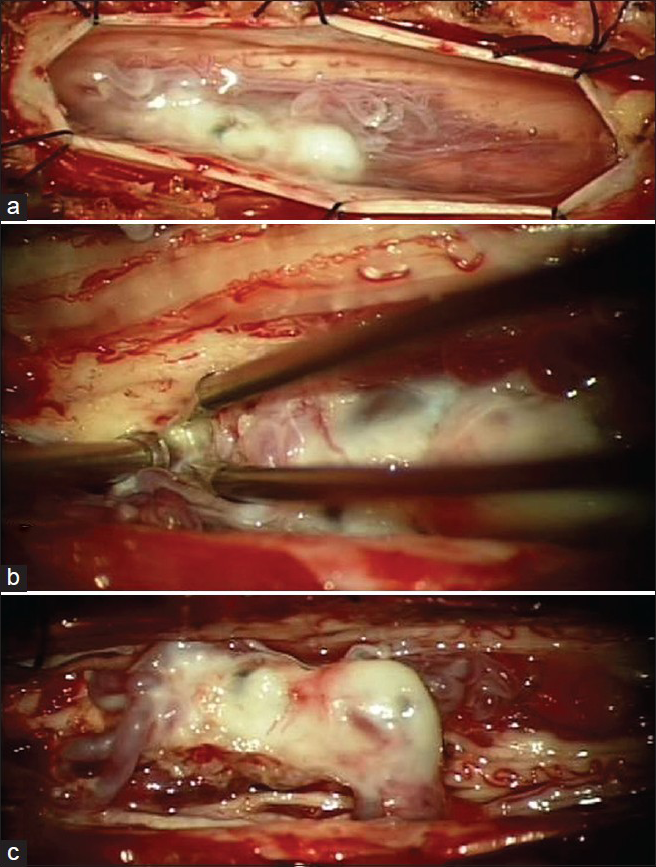
A 51-year-old female was admitted for progressive ascending spastic paraparesis and hypesthesia of 6 months’ duration extending from her feet to her buttocks. Neurologic examination revealed that light-touch sensation below T10 was impaired and that muscle strength was Medical Research Council grade 4 in both lower limbs. Additional myelopathy symptoms included bilateral extensor plantar response, intensified deep tendon reflexes in both knees and Achilles tendons, and fecal and urinary incontinence. A thoracic spinal lesion was suspected. Magnetic resonance imaging (MRI) was performed on the thoracic spine and showed a perimedullary vascular lesion [
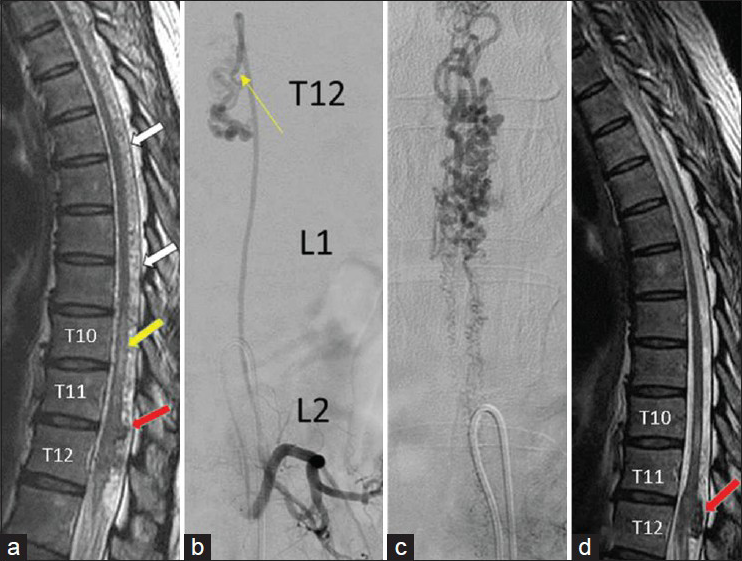
Figure 1
(a) T2WI MRI showing a hypointense lesion (red arrow) behind the spinal cord at T11 and T12 with peripheral tube-like lesions (white arrow), fistulas, and perimedullary venous drainage. At the T10 level (yellow arrow), it shows a hyperintense signal, indicating cord edema related to venous hypertension. (b) Angiogram showing the major feeding artery from the left L2 lumbar artery and a fistula at the T12 level. (c) Venous phase showing perimedullary venous drainage. (d) MRI taken after surgery showing decreased lesion size, cord edema, and vascularity compared with the previous MRI
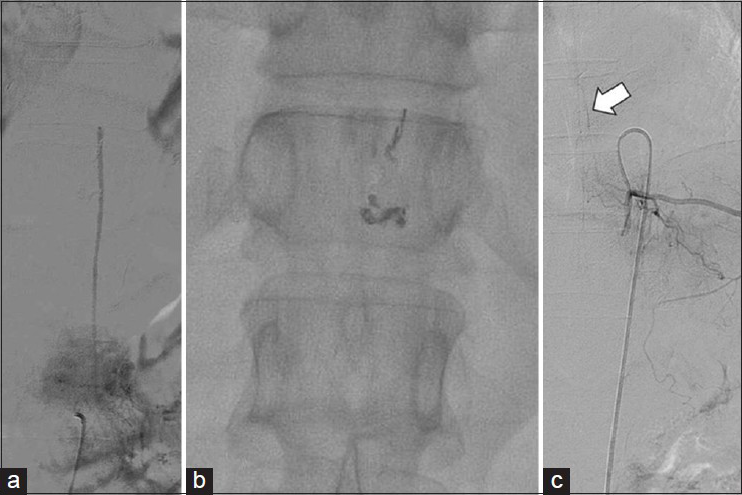
The symptoms improved immediately, and only residual foot numbness remained. To prevent spinal cord swelling, dexamethasone (4 mg every 8 h) was administered by intravenous drip for 1 week. Unfortunately, myelopathy symptoms came back gradually beginning 1 week after embolization and had returned to their pretreatment status 2 months later. Accordingly, we suggested follow-up catheter angiography to screen for vascular remodeling, but the patient refused it. Instead, we performed thoracic spine MRI to rule out the possibility that residual AV shunting was causing recurrent venous hypertension and cord hypoperfusion. The lesion still showed vascularity and edema, although more less than on the previous MRI [
After the operation, the patient's muscle strength returned to normal, her incontinence disappeared, and her sensation almost completely recovered. We followed up this patient for 6 months and noted no further symptoms except mild residual left leg numbness.
DISCUSSION
In patients with a perimedullary AVF, venous hypertension is the main cause of neurological dysfunction.[
According to Natarajan et al.,[
On the basis of our treatment experience, we propose that initial embolization, followed by surgery 5 days later to remove occult varices, is the best treatment strategy for AVF type IV-B AVF. Performing embolization first can decrease operation blood loss and duration. If significant vascularity remains after embolization, antiplatelet drugs should be administered to prevent excessive venous thrombosis. Steroid therapy and surgery must be implemented as soon as possible after embolization to forestall potential chemical injury to nerves, secondary mass effect, and neovascular generation.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
1. Cho WS, Kim KJ, Kwon OK. Clinical features and treatment outcomes of the spinal arteriovenous fistulas and malformations. J Neurosurg Spine. 2013. 19: 207-16
2. Dumont AS, Oldfield EH.editors. Spinal vascular malformation. Youmans Neurological Surgery. Philadelphia: Saunders Elsevier; 2011. 4: 4167-8
3. Ferch RD, Morgan MK, Sears WR. Spinal arteriovenous malformations: A review with case illustrations. J Clin Neurosci. 2001. 8: 299-304
4. Gueguen B, Merland JJ, Riche MC. Vascular malformations of the spinal cord: Intrathecal perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 1987. 37: 969-79
5. Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: Arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006. 59: S195-201
6. Natarajan SK, Born D. Histopathological changes in brain arteriovenous malformations after embolization using Onyx or N-butyl cyanoacrylate. J Neurosurg. 2009. 111: 105-13