- Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA
Correspondence Address:
Kimberly A. Foster
Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA
DOI:10.4103/2152-7806.178522
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Foster KA, Deibert CP, Choi PA, Gardner PA, Tyler-Kabara EC, Engh JA. Endoscopic third ventriculostomy as adjunctive therapy in the treatment of low-pressure hydrocephalus in adults. Surg Neurol Int 10-Mar-2016;7:26
How to cite this URL: Foster KA, Deibert CP, Choi PA, Gardner PA, Tyler-Kabara EC, Engh JA. Endoscopic third ventriculostomy as adjunctive therapy in the treatment of low-pressure hydrocephalus in adults. Surg Neurol Int 10-Mar-2016;7:26. Available from: http://surgicalneurologyint.com/surgicalint_articles/endoscopic-third-ventriculostomy-as-adjunctive-therapy-in-the-treatment-of-low%e2%80%91pressure-hydrocephalus-in-adults/
Abstract
Background:Treatment of low-pressure hydrocephalus (LPH) may require prolonged external ventricular drainage (EVD) at sub-zero pressures to reverse ventriculomegaly. Endoscopic third ventriculostomy (ETV) has been used in the treatment of noncommunicating hydrocephalus; however, indications for ETV are expanding.
Methods:Patients with the diagnosis of LPH as defined by the Pang and Altschuler criteria who underwent sub-zero drainage treatment over an 8-year period were included. Patients were divided into two cohorts based on whether or not ETV was employed during their treatment. Time from EVD placement to internalization of shunt was recorded for both groups; time from ETV to placement of shunt was recorded for the patients undergoing ETV.
Results:Sixteen adult patients with LPH were managed with sub-zero drainage method. Ten (62.5%) patients did not undergo ETV and the average time from first ventriculostomy to shunting was 73 days (range 14–257 days). Six (37.5%) patients underwent ETV during the course of their treatment; average time from initial ventriculostomy to shunt was 114 days (range 0–236 days) (P = 0.16). Time from development of LPH to ETV ranged from 28 days to 6.5 months. In the ETV group, of the 4 patients who underwent shunting, the average time to shunting following ETV was 15.25 days.
Conclusions:ETV can be used successfully in the management of refractory LPH to decrease the duration of EVD.
Keywords: Adult hydrocephalus, endoscopic third ventriculostomy, intracranial pressure, low-pressure hydrocephalus, shunt, subarachnoid space
INTRODUCTION
As initially described by Dandy, hydrocephalus (HCP) was defined as obstructive or communicating and typically associated with elevated intracranial pressure (ICP).[
Pang and Altschuler reported 12 patients with a “full-blown” hydrocephalic syndrome who had all been previously managed by medium-pressure shunt systems; patients presented with headache, lethargy, obtundation, and cranial neuropathies but were found to have low or low-normal ICP. Nearly, all patients required prolonged external ventricular drainage (EVD) at negative pressure to reverse the ventriculomegaly and symptoms of HCP. Importantly, symptoms throughout the period of EVD drainage correlated with ventricular size only, not ICP. Their diagnostic criteria for LPH included: (1) Neurologic decline at normal EVD or shunt pressures; (2) ventriculomegaly; (3) persistence of ventriculomegaly with ICP in the normal to low-normal range; and (4) clinical and radiographic response to sub-zero drainage. This method consisted of prolonged subatmospheric pressure drainage of the ventricular system until the ventricular shrinkage was maintained at a positive pressure, and a new shunt system could be inserted.
Endoscopic third ventriculostomy (ETV) has been employed successfully in the treatment of HCP in specific populations, particularly those patients with what the literature commonly refers to as the “noncommunicating” form of the disease. Despite the traditional concept that ETV is best utilized in these patients, other uses for ETV have been suggested. We report on the successful application of ETV in the treatment of patients with LPH.
METHODS
Patients
The authors performed a retrospective analysis of all adult inpatients admitted to the neurosurgical service at the University of Pittsburgh Medical Center between June 2005 and November 2013 to identify those patients who presented with LPH or developed this condition throughout their hospitalization. To meet inclusion criteria, patients with LPH were identified as those with (1) radiographic evidence of ventriculomegaly; (2) neurologic decline from baseline; (3) neurologic deficit despite conformation of normal or low ICP in the setting of a patent, functional EVD or ventricular shunt; and (4) clinical and radiographic response to the sub-zero drainage method. Within this group of patients, those who underwent ETV during the course of their treatment at the time an EVD was open and functional were identified. This review of patient data was approved by the Institutional Review Board.
Treatment of low-pressure hydrocephalus
All patients with LPH were treated with the sub-zero drainage method via external ventriculostomy. The EVD was initiated at a subatmospheric pressure, placed at a height ranging from 5 to 10 cmH2O below midbrain, and incrementally raised 1–2 cmH2O every 3–5 days. Prior to elevating the drain, a computed tomography (CT) scan was obtained to document stable or smaller ventricular caliber. If the patient demonstrated neurologic decline, the drain was again lowered to a more negative pressure and the cycle reinitiated. Patients with LPH underwent ETV while the EVD was still at a subatmospheric pressure. Patients underwent shunt placement once they demonstrated clinical and radiographic stability with an EVD leveled at or above midbrain.
Endoscopic third ventriculostomy
ETV was performed via a curvilinear skin incision in the right frontal region, with a single coronal burr hole placed at the mid-pupillary line. A small durotomy was created with electrocautery and scalpel, and a sheath was passed until CSF was obtained from the frontal horn of the lateral ventricle. Next, a rigid neuroendoscope was introduced and intraventricular anatomical landmarks were identified. A Bentson wire was used to create an ostomy in the floor of the third ventricle anterior to the mammillary bodies and a 2.0 French Fogarty balloon was inflated to increase the size of the ostomy. CSF flow was assessed and choroid plexus cauterization (CPC) was performed in some patients. Finally, the patient was then shunted at the time of ETV ± CPC or an EVD was replaced at the conclusion of the ETV, based on surgeon preference.
RESULTS
We identified 16 adult patients who met criteria necessary to obtain the diagnosis of LPH over the 8-year period in this study. All patients were managed with the sub-zero drainage method. Ten (62.5%) patients did not undergo ETV in the course of their treatment. All 10 (100%) patients in the non-ETV cohort required permanent shunting and the average time from placement of first ventriculostomy to shunting procedure in this group was 73 days (range 14–257 days).
Six (37.5%) patients underwent ETV during the course of their treatment; 4 were male and age ranged from 28 to 48 years. Two patients developed LPH following CSF diversion for HCP with elevated ICP secondary to tumor; one patient had a pineal germinoma causing obstructive HCP and the other patient developed communicating HCP secondary to a suprasellar cistern epidermoid resected via the endoscopic endonasal approach complicated by intraoperative rupture and postoperative CSF leak. LPH occurred in 2 patients with prior aneurysmal SAH requiring permanent CSF diversion with shunting at the time of their initial admission. Two patients had a history of prior shunt placement many years before presenting with LPH. Features of the patients are shown in
Clinical presentation was highly variable with signs and symptoms including headache, lethargy, seizure, upgaze restriction, and coma. No patients presented with autonomic instability or cardiac arrhythmias, as has been reported in other LPH series.[
Five patients underwent ETV while the EVD was at a subatmospheric pressure (≤0 cmH2O); 1 patient had a ventriculoperitoneal/pleural shunt in place at the time of ETV, which was removed temporarily for ETV and then replaced at the conclusion of the operation. Time from development of LPH to ETV ranged from 28 days to 6.5 months. Following ETV, the clinical course was highly variable. One patient underwent rapid EVD wean (in 8 days) and did not require shunting. Two patients underwent shunting at the time of ETV and have not demonstrated shunt failure to date. One patient was shunted 16 days after ETV. Two patients were deemed by the operative surgeon to have an unsuccessful ETV at the time of surgery; of these two, 1 patient died 2 months after ETV with an EVD still at a subatmospheric pressure, and the other proceeded to shunt internalization following another 1.5 months of sub-zero management post-ETV.
The average time from placement of initial ventriculostomy to shunt in this group was 114 days (range 0–236 days). When comparing the ETV and non-ETV cohorts, the overall timing between first ventriculostomy and shunting did not reach statistical significance (P = 0.16). However, of the 4 patients in the ETV cohort who underwent shunting, the average time to shunting following ETV was 15.25 days (range 0–45 days). Of the entire study group, 1 patient avoided shunting altogether and this occurred following ETV. There were no procedure-related complications from ETV or shunt placement.
Illustrative case
A 31-year-old male with a medical history significant for Ehlers–Danlos type IV and smoking initially presented at age 24 with Hunt Hess Grade 4 aneurysmal SAH and underwent craniotomy for evacuation of intraparenchymal clot and clipping of a carotid bifurcation aneurysm. He was managed with an EVD and subsequently underwent shunt internalization with a medium-pressure programmable valve. Initially with hemiplegia, he recovered well neurologically, regained all function and returned to his prior level of function. Three years later, he underwent elective coiling of a carotid wall aneurysm and at age 30 elected for an open craniotomy for clipping of ophthalmic and posterior communicating artery aneurysms. Throughout this time, he did not have evidence of shunt failure and required no revisions.
In the month prior to his diagnosis of LPH, he presented with headaches, and a shunt tap revealed elevated ICP, prompting shunt exploration and proximal catheter revision. A few weeks later, he presented with lethargy; CT revealed significant pan-ventriculomegaly with transependymal absorption and abdominal imaging showed an abdominal pseudocyst. He underwent removal of shunt and placement of EVD. CSF analysis at the time of shunt externalization revealed an Enterobacter infection and the patient completed a 3 weeks course of meropenem with resolution of his CSF infection. Of note, his course was also complicated by electrographic evidence of epileptiform discharges, managed by phenytoin.
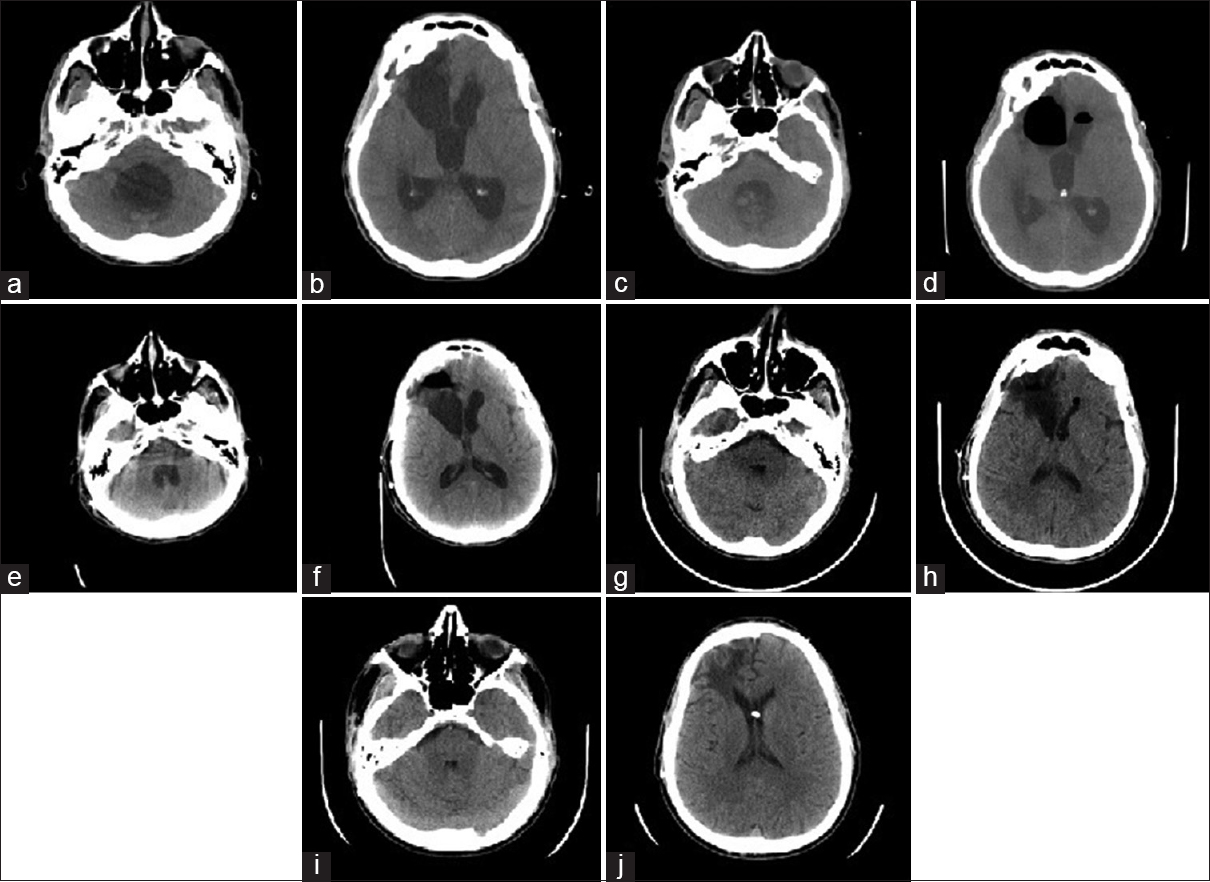
At this time, it was found that he did not drain CSF at a positive pressure and so was managed with the sub-zero drainage method. His lowest EVD pressure was −12 cmH2O. On EVD day 32, with EVD pressure of −5 cmH2O, he underwent ETV with CPC (imaging 3 h prior to procedure shown in Figure
Figure 1
Axial computed tomography image showing ventriculomegaly of the fourth ventricle (a) and lateral ventricles (b) obtained 3 h prior to endoscopic third ventriculostomy/choroid plexus cauterization, with external ventricular drain positioned at 5 cmH2O below midbrain. Computed tomography image obtained immediately following endoscopic third ventriculostomy/choroid plexus cauterization of the fourth ventricle (c) and lateral ventricles (d). Computed tomography image obtained 3 h prior to placement of ventriculopleural shunt, with external ventricular drain at 5 cmH2O above midbrain, showing fourth (e) and lateral ventricles (f). Computed tomography imaging obtained on postoperative day 2 from shunting (g and h) and at 3 months neurosurgical follow-up (i and j)
DISCUSSION
LPH is a rare form of HCP consisting of neurologic decline and significant ventriculomegaly in the setting of low ICP. Review of literature since the landmark paper of Pang and Altschuler revealed nearly 70 reported cases of LPH. Many of these cases have been summarized in the work of Akins et al. and, in addition, the authors reported on 9 of their own patients.[
LPH is associated with multiple conditions including SAH, tumor, and chronic HCP. Akins et al. noted a tendency for pathology isolated to the posterior fossa, including fourth ventricular tumors, hemorrhage, and basilar meningitis. The occurrence of LPH following lumbar puncture has been reported in a small case series of shunted patients who subsequently developed shunt failure due to LPH and an intraventricular pressure too low to open the shunt valve.[
Multiple theories attempt to explain the underlying pathophysiology of LPH. Early work to model HCP, including the contributions of Hakim et al., suggested that the brain should be viewed as a porous sponge that obeyed the principles of viscoelastic materials.[
Another model of LPH was based on the principle of hysteresis, as proposed by Lesniak et al.[
The model of Akins et al. differs from the prior hypotheses and proposes the brain to be like a boggy sponge in LPH, in contrast to the “wrung-out” cortical mantle of other theories.[
Rekate et al. emphasized the importance of taking into account all compartments of CSF, which includes the ventricles, brain parenchyma, spinal subarachnoid space (SSAS), and the often neglected cortical subarachnoid space (CSAS).[
As illustrated by Akins et al., movement of CSF in and out of the brain is critical in the development of LPH and can be explained by Darcy's law, which describes the movement of fluid in and out of a porous substance.[
Permeability of the brain and viscosity of CSF also play an important role in LPH. If the permeability of the brain is increased, so will the movement of CSF. In LPH, it has been hypothesized that the permeability (k) of the brain itself is deranged, as evidenced by the presence of transependymal absorption seen commonly in imaging studies of patients with LPH.[
The role of endoscopic third ventriculostomy
ETV has generally been employed in the treatment of noncommunicating HCP. The ETV Success Score (ETVSS) is a recently designed, externally validated tool to guide the selection of patients for ETV.[
To date, there is a little attention in literature given to the use of ETV in the treatment paradigm of LPH. Rekate et al. reports on 3 total patients who required sub-zero drainage for LPH and were treated with ETV; 1 patient still required placement of a shunt but no longer needed cervical wrapping following ETV, another managed with ETV alone[
In our LPH population, patients required prolonged hospitalizations for protracted sub-zero EVD, often on the order of months, before an atmospheric pressure could be obtained and a shunt could be placed. Our goal was to employ ETV to decrease the amount of time an EVD was required and allow for earlier shunting in adult patients who had developed LPH. There was no statistically significant difference in the overall time from ventriculostomy to shunt placement (or wean) between those patients undergoing ETV and those who did not. However, this is due to an inherent bias to perform ETV on patients who were refractory to sub-zero drainage and, importantly, all but 1 patient undergoing ETV was at sub-zero (one at zero) pressures at the time of ETV. Conversely, patients who demonstrated response to drainage were not readily considered for ETV. The difference in the clinical courses of the two cohorts should be highlighted post-ETV, as the rapid return to shunting occurred in this time period for those patients undergoing ETV (mean time to shunt was approximately 2 weeks). Moreover, only in the ETV cohort did any patient avoid a permanent shunt. ETV did not allow rapid shunting in all patients and, admittedly, we need to refine our ability to predict which patients in our LPH cohort would benefit from ETV.
This study has multiple limitations. It is a retrospective review and suffers from the inherent biases as such; a prospective, randomized evaluation of patients with LPH managed with and without ETV would definitively address the utility of ETV, if any, in this population. The most important bias in our study is selection bias. Throughout the time of this study, patients with LPH were managed by multiple different physicians and surgeons. The neurosurgical subspecialist treating the original neurosurgical problem (tumor, aneurysm, etc.,) may or may not have cared for the patient throughout their LPH state and, given the very long clinical course of most patients, many were managed by different physicians and care teams. Thus, the decision to perform ETV was based on attending physician preference and, admittedly at our institution, the comfort with performing ETV and the acceptance of ETV indications are variable and surgeon-dependent. Selection bias also explains why some patients remained in the non-ETV cohort (these patients were not considered for ETV), despite very long hospitalizations. Moreover, we do not know what may have happened to those patients in the ETV cohort had they never undergone ETV.
In the patient who remained shunt-free after ETV, our imaging modalities (cranial CT and MRI) demonstrated that the patient had radiographic communicating HCP (all ventricles demonstrated the same extent of ventriculomegaly). While ETV is a well-accepted modality in noncommunicating (obstructive) HCP, we are suggesting it has value in the communicating form of the disease we defined our LPH patients as communicating. The definitions of communicating and noncommunicating HCP are debated, and a deeper understanding of anatomical site of abnormality is needed (as discussed earlier, all HCP may be obstructive), and a full discussion is beyond the scope of this manuscript.
CONCLUSIONS
ETV can be used successfully in the management of patients with refractory LPH. The benefit of ETV in the setting of LPH may be based on the concept of a pressure gradient, not an absolute intraventricular pressure, which exists across the cortical mantle in patients with altered brain viscoelastic properties. Ultimately, ETV could normalize (decrease) this pressure gradient and bring the important CSAS into communication with the ventricular CSF space. While ETV alone may not be sufficient in the treatment of LPH in all patients, it may allow for earlier shunting, or even a decrease in the number of shunt-dependent patients. Future investigations will need to clarify the appropriate timing of ETV and subsequent shunting, as well as identify those patients who will benefit most from ETV in the treatment of LPH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Commentary
Hydrocephalus is very problematic and very difficult problem to treat and still to know many things for a simple, comprehensive solution of it. This statement is truer in cases of low-pressure hydrocephalus (LPH).
Though obstructive hydrocephalus is the main indication of endoscopic third ventriculostomy (ETV), it is now using in different types of selective communicating hydrocephalus.[
Commentary
In this well written article, the authors described their experiences with the surgical management of low-pressure hydrocephalus, which is a very rare, complicated, and difficult condition. Their surgical management is mainly based on endoscopic third ventriculostomy (ETV), after sub-zero drainage. I do agree that in the hard clinical situation with LPH, when simple shunt is highly possible to be ineffective, ETV can be a good alternative for the management of hydrocephalus, either for short or longer period of time. However, the result of this article is not persuasive enough. In this retrospective, small sample size study from experiences of one single center, totally 16 patients were enrolled. Among them, 10 cases had sub-zero external ventricular drainage (EVD), followed by shunt, while the rest 6 cases had EVD, ETV and shunt (4 cases). In both groups (with or without ETV), the LPH was eventually successfully managed. For those 4 cases who had ETV and shunt, the time between ETV and shunt is significantly shorter than those 10 cases with only EVD and shunt. However, if we compare the time between initial EVD and final shunt of two groups (with/without ETV), the period of time is slightly longer in the ETV group, but the P value is 0.16. This result is very difficult to be interpreted. The small sample size may be a possible reason. But other reasons should be further analyzed with more accumulated cases and data.
LPH is a very complicated condition. Its etiology is still controversial. The authors proposed that low brain elasticity may probably play an important role, because water within the brain parenchyma would be forced out and into the ventricular system. This mechanism is still worth further exploration. But to me, another possible mechanism, which was already proved by animal model in normal pressure hydrocephalus (NPH), appears more convincing. Di Rocco et al. reported experimental hydrocephalus induced by mechanical increment of intraventricular pulse pressure.[
After all, in this small sample size study, ETV appears to be a possible alternative to manage LPH. The potential mechanism is still not clear and needs further research.
References
1. Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure. A treatable syndrome. N Engl J Med. 1965. 273: 117-26
2. Akins PT, Guppy KH, Axelrod YV, Chakrabarti I, Silverthorn J, Williams AR. The genesis of low pressure hydrocephalus. Neurocrit Care. 2011. 15: 461-8
3. Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: A review of decompressive craniectomy management. Neurocrit Care. 2008. 9: 269-76
4. Clarke MJ, Maher CO, Nothdurft G, Meyer F. Very low pressure hydrocephalus. Report of two cases. J Neurosurg. 2006. 105: 475-8
5. Dandy WE, Blackfan KD. An experimental and clinical study of internal hydrocephalus. J Am Med Assoc. 1913. 61: 2216-7
6. Dandy WE, Blackfan KD. Internal hydrocephalus: Second paper. Am J Dis Child. 1917. 14: 424-43
7. Daniel RT, Lee GY, Halcrow SJ. Low-pressure hydrocephalic state complicating hemispherectomy: A case report. Epilepsia. 2002. 43: 563-5
8. Darcy H.editorsThe Public Fountains of the City of Dijon: Exposure and Application. Paris: Victor Dalmont; 1856. p.
9. Dias MS, Li V, Pollina J. Low-pressure shunt ‘malfunction’ following lumbar puncture in children with shunted obstructive hydrocephalus. Pediatr Neurosurg. 1999. 30: 146-50
10. Durnford AJ, Kirkham FJ, Mathad N, Sparrow OC. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus: Validation of a success score that predicts long-term outcome. J Neurosurg Pediatr. 2011. 8: 489-93
11. Filippidis AS, Kalani MY, Nakaji P, Rekate HL. Negative-pressure and low-pressure hydrocephalus: The role of cerebrospinal fluid leaks resulting from surgical approaches to the cranial base. J Neurosurg. 2011. 115: 1031-7
12. Hailong F, Guangfu H, Haibin T, Hong P, Yong C, Weidong L. Endoscopic third ventriculostomy in the management of communicating hydrocephalus: A preliminary study. J Neurosurg. 2008. 109: 923-30
13. Hakim S, Venegas JG, Burton JD. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: Mechanical interpretation and mathematical model. Surg Neurol. 1976. 5: 187-210
14. Hamilton MG, Price AV. Syndrome of inappropriately low-pressure acute hydrocephalus (SILPAH). Acta Neurochir Suppl. 2012. 113: 155-9
15. Kulkarni AV, Riva-Cambrin J, Browd SR. Use of the ETV success score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J Neurosurg Pediatri. 2011. 7: 143-6
16. Lesniak MS, Clatterbuck RE, Rigamonti D, Williams MA. Low pressure hydrocephalus and ventriculomegaly: Hysteresis, non-linear dynamics, and the benefits of CSF diversion. Br J Neurosurg. 2002. 16: 555-61
17. Oertel JM, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR. Endoscopic third ventriculostomy for obstructive hydrocephalus due to intracranial hemorrhage with intraventricular extension. J Neurosurg. 2009. 111: 1119-26
18. Owler BK, Jacobson EE, Johnston IH. Low pressure hydrocephalus: Issues of diagnosis and treatment in five cases. Br J Neurosurg. 2001. 15: 353-9
19. Paidakakos N, Borgarello S, Naddeo M.editors. Indications for endoscopic third ventriculostomy in normal pressure hydrocephalus. Acta Neurochirurgica Supplement. Dusseldorf: Springer; 2012. p. 123-7
20. Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. 1994. 35: 643-55
21. Rangel-Castilla L, Barber S, Zhang YJ. The role of endoscopic third ventriculostomy in the treatment of communicating hydrocephalus. World Neurosurg. 2012. 77: 555-60
22. Rekate HL, Nadkarni TD, Wallace D. The importance of the cortical subarachnoid space in understanding hydrocephalus. J Neurosurg Pediatr. 2008. 2: 1-11
23. Sacko O, Boetto S, Lauwers-Cances V, Dupuy M, Roux FE. Endoscopic third ventriculostomy: Outcome analysis in 368 procedures. J Neurosurg Pediatr. 2010. 5: 68-74
24. Shimizu T, Luciano MG, Fukuhara T. Role of endoscopic third ventriculostomy at infected cerebrospinal fluid shunt removal. J Neurosurg Pediatr. 2012. 9: 320-6
25. Teo C, Jones R. Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg. 1996. 25: 57-63
26. Vassilyadi M, Farmer JP, Montes JL. Negative-pressure hydrocephalus. J Neurosurg. 1995. 83: 486-90
27. Vogel TW, Bahuleyan B, Robinson S, Cohen AR. The role of endoscopic third ventriculostomy in the treatment of hydrocephalus. J Neurosurg Pediatr. 2013. 12: 54-61
28. Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: The importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 2011. 27: 1063-71
29. Warf BC, Stagno V, Mugamba J. Encephalocele in Uganda: Ethnic distinctions in lesion location, endoscopic management of hydrocephalus, and survival in 110 consecutive children. J Neurosurg Pediatr. 2011. 7: 88-93
30. Yadav YR, Parihar V, Agrawal M, Bhatele PR. Endoscopic third ventriculostomy in tubercular meningitis with hydrocephalus. Neurol India. 2011. 59: 855-60
31. Filippidis AS, Kalani MY, Nakaji P, Rekate HL. Negative-pressure and low-pressure hydrocephalus: The role of cerebrospinal fluid leaks resulting from surgical approaches to the cranial base. J Neurosurg. 2011. 115: 1031-7
32. Hamilton MG, Price AV. Syndrome of inappropriately low-pressure acute hydrocephalus (SILPAH). Acta Neurochir Suppl. 2012. 113: 155-9
33. Moorthy RK, Rajshekhar V. Endoscopic third ventriculostomy for hydrocephalus: A review of indications, outcomes, and complications. Neurol India. 2011. 59: 848-54
34. Rekate HL, Nadkarni TD, Wallace D. The importance of the cortical subarachnoid space in understanding hydrocephalus. J Neurosurg Pediatr. 2008. 2: 1-11
35. Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. Experimental hydrocephalus following mechanical increment of intraventricular pulse pressure. Experientia. 1977. 33: 1470-2