- Department of Pathology and Laboratory Medicine, Miller School of Medicine, University of Miami, Miami, FL, USA
- Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA
- Department of Neurological Surgery, Miller School of Medicine, University of Miami, Miami, Florida, USA
- Aventura, Florida, USA
Correspondence Address:
Kirill A. Lyapichev
Department of Neurological Surgery, Miller School of Medicine, University of Miami, Miami, Florida, USA
DOI:10.4103/2152-7806.185783
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lyapichev KA, Bregy A, Cassel A, Handfield C, Velazquez-Vega J, Kay MD, Basil G, Komotar RJ. Glioblastoma multiforme of the optic chiasm: A rare case of common pathology. Surg Neurol Int 07-Jul-2016;7:
How to cite this URL: Lyapichev KA, Bregy A, Cassel A, Handfield C, Velazquez-Vega J, Kay MD, Basil G, Komotar RJ. Glioblastoma multiforme of the optic chiasm: A rare case of common pathology. Surg Neurol Int 07-Jul-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/glioblastoma-multiforme-optic-chiasm-rare-case-common-pathology/
Abstract
Background:Malignant optic and chiasmatic gliomas are extremely rare, and are classified pathologically as anaplastic astrocytoma or glioblastoma multiforme (GBM). Approximately 40 cases of optic GBM in adults have been reported in the literature, and only five of them were described to originate from the optic chiasm.
Case Description:An 82-year-old male patient with a past medical history of diabetes mellitus type 2, melanoma, and bladder cancer presented with gradual vision loss of the left eye in a period of 1 month. After neuro-ophthalmological examination, the decision of thither magnetic resonance imaging (MRI) studies was made. It showed a contrast enhancing mass in the region of the optic chiasm. In this case, imaging study was not enough to establish an accurate diagnosis and a left pterional craniotomy for biopsy and resection of the optic chiasmal mass was performed. After histological evaluation of the mass tissue, the diagnosis of GBM was made. Taking into account the patient's poor condition and unfavorable prognosis he was moved to inpatient hospice. The patient deceased within 2 months after surgery.
Conclusion:Chiasmal GBM is an extremely rare condition where a biopsy is necessary for accurate diagnosis and optimal treatment. Differential diagnosis for such lesions can be very difficult and include demyelinating optic neuritis and non-demyelinating inflammatory optic neuropathy (e.g., sarcoid), vascular lesions (e.g., cavernoma), compressive lesions of the optic apparatus, metastatic malignancy, and primary tumors of the anterior optic pathway. The role of chemotherapy and radiotherapy including novel stereotaxic radiosurgery methods is still unclear and will need to be evaluated.
Keywords: Brain tumor, glioblastoma, glioma
INTRODUCTION
Optic nerve gliomas represent approximately 2% of all brain tumors.[
CASE PRESENTATION
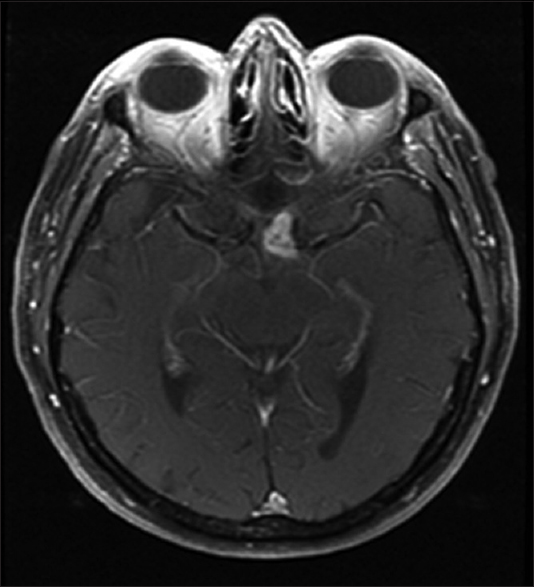
An 82-year-old man with an extensive medical history including chronic obstructive pulmonary disease, type 2 diabetes, coronary artery disease, history of melanoma, and previously treated bladder cancer presented with 1 month of rapid onset visual loss in the left eye. It manifested initially as a blurry vision, which progressed to severe loss of vision in the left eye. Neuro-ophthalmological examination showed a left relative afferent pupillary defect, which was suggestive of either an optic neuropathy or diffuse retinal pathology. Formal visual field testing demonstrated severe generalized depression in the left eye and a superotemporal quadrantic defect in the right eye indicating pathology affecting the left optic nerve and chiasm. Subsequent magnetic resonance imaging (MRI) of the brain confirmed a contrast enhancing mass in the region of the left-side of the optic chiasm extending from the suprasellar cistern into the left optic nerve [
The differential diagnosis for tumors affecting the anterior visual pathway is myriad because a vast majority (84%) of patients present with visual impairment and no other ophthalmologic or neurologic symptoms. Considerations should include demyelinating optic neuritis and non-demyelinating inflammatory optic neuropathy (e.g., sarcoid), vascular lesions (e.g., cavernoma), compressive lesions of the optic apparatus, metastatic infiltration particularly in the setting of prior known systemic malignancy as in our case with a history of metastatic melanoma, and primary tumors of the anterior optic pathway.[
Given the patient's visual loss and the uncertain etiology of the lesion, a left pterional craniotomy for biopsy and resection of the optic chiasmal mass was performed.
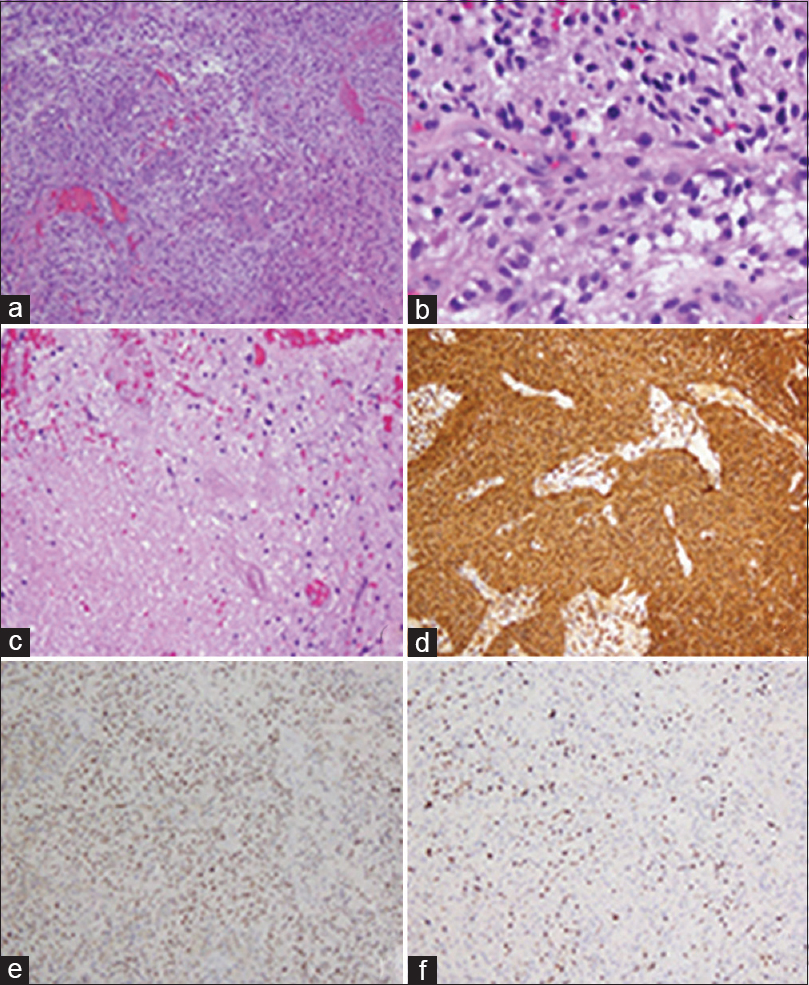
On gross pathology, the tumor was a light-tan, irregularly-shaped soft tissue fragment measuring 0.8 × 0.3 × 0.2 cm in aggregate. On histological evaluation, the tumor was composed of glial cells with pleomorphic nuclei and areas of vascular proliferation and necrosis. Immunohistochemistry was positive for glial fibrillary acidic protein (GFAP) and p53 and showed a Ki 67 proliferation index of ~10% [
Figure 2
A highly cellular astrocytic tumor is seen with marked microvascular proliferation (a), mitotic activity (b) and areas of necrosis (c). The tumor was strongly immunoreactive to glial fibrillary acidic protein immunohistochemistry (d) and the tumor nuclei were positive for p53 (e). The ki-67 proliferation index was estimated to be approximately 10% (f)
Postoperatively, the patient felt fine and did not show any neurological impairment. No postoperative radiation or chemotherapy was administered, however, 6 days post-discharge he started to show confusion, lethargy, and unsteady gait, and was readmitted to the hospital. A lumbar puncture (LP) was performed to rule out any infectious etiology. The patient's condition and treatment options were discussed and decision on transferring him to inpatient hospice was made. The patient deceased within 2 months after surgery.
DISCUSSION
Since the optic GBM infiltrates extensively and grows rapidly, the prognosis is unfavorable. Reported survival ranges from 6–14 months after diagnosis. Dinh et al. published a case of chiasmatic GBM where the patient survived 14 months after onset of the symptoms, which is the longest survival of such patients in the literature.[
As generally known for GBM, optic nerve GBM also shows a slight preference for males (male to female ratio is 1.38:1) and cases are reported from 22 years old to 79 years old patients.[
Although glioblastoma is a histopathologic diagnosis, novel molecular studies using the cancer genome atlas (TCGA) have identified multiple tumor subtypes providing us much more than just diagnostic information.[
CONCLUSION
Our case represents a GBM that originated in the chiasm and then infiltrated into left optic nerve with subsequent vision impairment. There were no other lesions arising from secondary structures. Differential diagnosis for such lesions can be very difficult and biopsy may be necessary for diagnosis and optimal treatment. The role of chemotherapy and radiotherapy including novel stereotaxic radiosurgery methods is still unclear and will need to be evaluated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alvord EC, Lofton S. Gliomas of the optic nerve or chiasm. Outcome by patients’ age, tumor site, and treatment. J Neurosurg. 1988. 68: 85-98
2. Ashur-Fabian O, Blumenthal DT, Bakon M, Nass D, Davis PJ, Hercbergs A. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: A case report and review of the literature. Anticancer Drugs. 2013. 24: 315-23
3. Caignard A, Faguer R, Mercier P, Menei P, Milea D. Optic nerve and visual pathways primary glioblastoma treated with radiotherapy and temozolomide chemotherapy. Eur J Ophthalmol. 2014. 24: 637-40
4. Darefsky AS, Dubrow R. International variation in the incidence of adult primary malignant neoplasms of the brain and central nervous system. Cancer Causes Control. 2009. 20: 1593-1604
5. Dimitrov L, Hong CS, Yang C, Zhuang Z, Heiss JD. New developments in the pathogenesis and therapeutic targeting of the IDH1 mutation in glioma. Int J Med Sci. 2015. 12: 201-13
6. Dinh TT, Wang YY, Rosenfeld JV, Cherny M. Glioblastoma of the optic chiasm. J Clin Neurosci. 2007. 14: 502-5
7. Hartel PH, Rosen C, Larzo C, Nestor S. Malignant optic nerve glioma (glioblastoma multiforme): A case report and literature review. W J Med J. 2006. 102: 29-31
8. Lalezari S, Chou AP, Tran A, Solis OE, Khanlou N, Chen W. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol. 2013. 15: 370-81
9. Matloob S, Fan JC, Danesh-Meyer HV. Multifocal malignant optic glioma of adulthood presenting as acute anterior optic neuropathy. J Clin Neurosci. 2011. 18: 974-7
10. Reithmeier T, Graf E, Piroth T, Trippel M, Pinsker MO, Nikkhah G. BCNU for recurrent glioblastoma multiforme: Efficacy, toxicity and prognostic factors. BMC Cancer. 2010. 10: 30-
11. Veliz I, Loo Y, Castillo O, Karachaliou N, Nigro O, Rosell R. Advances and challenges in the molecular biology and treatment of glioblastoma-Is there any hope for the future?. Ann Transl Med. 2015. 3: 7-
12. Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010. 17: 98-110