- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
- Department of Neurology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Correspondence Address:
Ha Son Nguyen
Department of Neurology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
DOI:10.4103/2152-7806.166788
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Doan N, Rozansky G, Nguyen HS, Gelsomino M, Shabani S, Mueller W, Johnson V. Granulomatous amebic encephalitis following hematopoietic stem cell transplantation. Surg Neurol Int 07-Oct-2015;6:
How to cite this URL: Doan N, Rozansky G, Nguyen HS, Gelsomino M, Shabani S, Mueller W, Johnson V. Granulomatous amebic encephalitis following hematopoietic stem cell transplantation. Surg Neurol Int 07-Oct-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/granulomatous-amebic-encephalitis-following-hematopoietic-stem/
Abstract
Background:Granulomatous amebic encephalitis (GAE) is rare, but often fatal. The infection has been documented predominantly among the immunocompromised population or among those with chronic disease. To date, however, there have only been eight cases regarding the infection following hematopoietic stem cell transplantation (HSCT).
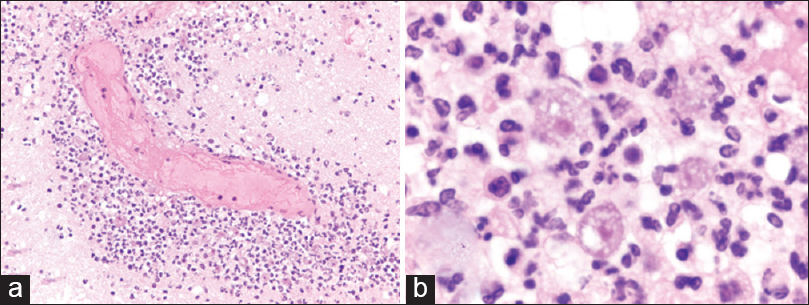
Case Description:A 62-year-old female with a history of relapsed diffuse large B-cell lymphoma, recently underwent peripheral blood autologous stem cell transplant after BEAM conditioning (day 0). On day +15, she began to exhibit worsening fatigue, generalized weakness, and fever. Symptoms progressed to nausea, emesis, somnolence, confusion, and frontal headaches over the next few days. Imaging demonstrated multifocal ill-defined vasogenic edema with patchy enhancement. The patient was started on broad antibiotics, antifungals, and seizure prophylaxis. Evaluation for bacterial, fungal, mycobacterial, and viral etiologies was fruitless. Her mental status progressively deteriorated. On day +22, she exhibited severe lethargy and went into pulseless electrical activity arrest, requiring chest compressions. The episode lasted Acanthamoeba GAE.
Conclusion:The authors report the third case of GAE after autologous stem cell transplant, and the ninth case overall after HSCT. This case is unusual due to its rapid clinical presentation after HSCT compared to prior literature. The case highlights the need for high suspicion of Acanthamoeba infection in this patient population.
Keywords: Acanthamoeba, encephalitis, stem cell transplantation
INTRODUCTION
Encephalitis is defined by acute inflammation of the brain, which may be attributed to an immunological or infectious process.[
GAE has been documented predominantly among the immunocompromised population or among those with chronic disease.[
CASE PRESENTATION
Patient is a 62-year-old female with history of relapsed diffuse large B-cell lymphoma (initially diagnosed 4 years ago s/p 6 cycles of R-CHOP followed by remission, with relapse 4 months ago s/p 3 cycles of RICE) who had recently underwent peripheral blood autologous stem cell transplant after BEAM conditioning (BiCNU, etoposide, Ara-c, melphalan). This was complicated by a neutropenic fever and hypotension, requiring Intensive Care Unit observation. An infectious work-up was negative, and the patient was discharged on day +11 with evidence of peripheral count engraftment.
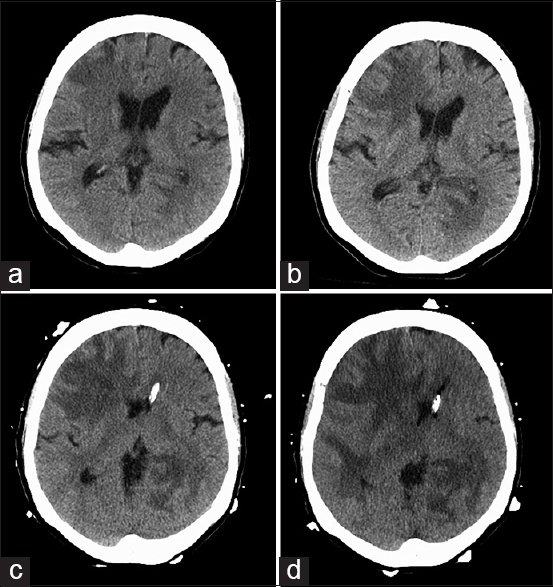
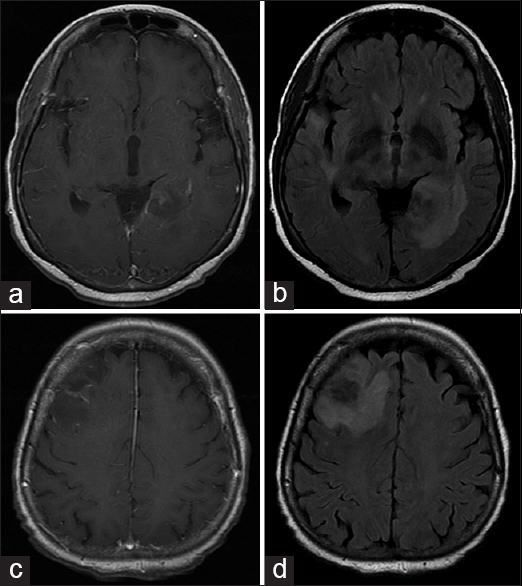
On day +15, she began to exhibit worsening fatigue, generalized weakness, and fever up to 100.9F. She was evaluated in the emergency department (ED), where subsequent chest X-ray, blood cultures, and urinalysis did not show any evidence of infection. Symptoms progressed to nausea, emesis, somnolence, confusion, and frontal headaches over the next few days. On day +18, the patient returned to the ED. The patient denied changes with vision, slurred speech, focal weakness, paresthesias, photo/phonophobia, or neck stiffness. A computed tomography (CT) head showed ill-defined hypodense areas with surrounding vasogenic edema in the right frontal lobe with subtle mass effect and effacement of the right frontal horn with similar ill-defined hypoattenuation in the left peri-atrial region and right temporal horn [
The patient was started on broad antibiotics, antifungals, and seizure prophylaxis. Evaluation for bacterial, fungal, mycobacterial, and viral etiologies was fruitless. Her mental status progressively deteriorated, but she was able to follow simple commands with effort. On day +22, she exhibited severe lethargy followed by pulseless electrical activity arrest, requiring chest compressions. The episode lasted <2 min and her pulse was restored. Two hours later, she exhibited seizure-like activity, which ultimately required emergent intubation for airway protection. A repeat CT head was stable [
DISCUSSION
Acanthamoeba is a type of free-living ameba that often resides in soil, air, or water environments.[
Including this case, there have been nine reported patients with GAE following HSCT, six allogeneic HSCT recipients, and three autologous HSCT recipients.[
CONCLUSION
The authors reported the third case of GAE after autologous stem cell transplant, and the ninth case overall after HSCT. This case is unusual due to its rapid clinical presentation after HSCT compared to prior literature. The case highlights the need for high suspicion of Acanthamoeba infection in this patient population, especially with the common prevalence of the organism in the environment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abd H, Saeed A, Jalal S, Bekassy AN, Sandström G. Ante mortem diagnosis of amoebic encephalitis in a haematopoietic stem cell transplanted patient. Scand J Infect Dis. 2009. 41: 619-22
2. Akpek G, Uslu A, Huebner T, Taner A, Rapoport AP, Gojo I. Granulomatous amebic encephalitis: An under-recognized cause of infectious mortality after hematopoietic stem cell transplantation. Transpl Infect Dis. 2011. 13: 366-73
3. Anderlini P, Przepiorka D, Luna M, Langford L, Andreeff M, Claxton D. Acanthamoeba meningoencephalitis after bone marrow transplantation. Bone Marrow Transplant. 1994. 14: 459-61
4. Castellano-Sanchez A, Popp AC, Nolte FS, Visvesvara GS, Thigpen M, Redei I. Acanthamoeba castellani encephalitis following partially mismatched related donor peripheral stem cell transplantation. Transpl Infect Dis. 2003. 5: 191-4
5. Feingold JM, Abraham J, Bilgrami S, Ngo N, Visvesara GS, Edwards RL. Acanthamoeba meningoencephalitis following autologous peripheral stem cell transplantation. Bone Marrow Transplant. 1998. 22: 297-300
6. Fung KT, Dhillon AP, McLaughlin JE, Lucas SB, Davidson B, Rolles K. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl. 2008. 14: 308-12
7. Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, Cossen CK. Beyond viruses: Clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006. 43: 1565-77
8. Gupta D, Panda GS, Bakhshi S. Successful treatment of Acanthamoeba meningoencephalitis during induction therapy of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008. 50: 1292-3
9. Kaul DR, Lowe L, Visvesvara GS, Farmen S, Khaled YA, Yanik GA. Acanthamoeba infection in a patient with chronic graft-versus-host disease occurring during treatment with voriconazole. Transpl Infect Dis. 2008. 10: 437-41
10. Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003. 16: 273-307
11. Maritschnegg P, Sovinz P, Lackner H, Benesch M, Nebl A, Schwinger W. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treated with multimodal antimicrobial therapy and hyperbaric oxygen. J Clin Microbiol. 2011. 49: 446-8
12. Parija SC, Dinoop K, Venugopal H. Management of granulomatous amebic encephalitis: Laboratory diagnosis and treatment. Trop Parasitol. 2015. 5: 23-8
13. Pemán J, Jarque I, Frasquet J, Alberola C, Salavert M, Sanz J. Unexpected postmortem diagnosis of Acanthamoeba meningoencephalitis following allogeneic peripheral blood stem cell transplantation. Am J Transplant. 2008. 8: 1562-6
14. Satlin MJ, Graham JK, Visvesvara GS, Mena H, Marks KM, Saal SD. Fulminant and fatal encephalitis caused by Acanthamoeba in a kidney transplant recipient: Case report and literature review. Transpl Infect Dis. 2013. 15: 619-26
15. Seijo Martinez M, Gonzalez-Mediero G, Santiago P, Rodriguez De Lope A, Diz J, Conde C. Granulomatous amebic encephalitis in a patient with AIDS: Isolation of Acanthamoeba sp. Group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J Clin Microbiol. 2000. 38: 3892-5
16. Singh P, Kochhar R, Vashishta RK, Khandelwal N, Prabhakar S, Mohindra S. Amebic meningoencephalitis: Spectrum of imaging findings. AJNR Am J Neuroradiol. 2006. 27: 1217-21
17. Stahl JP, Mailles A. What is new about epidemiology of acute infectious encephalitis?. Curr Opin Neurol. 2014. 27: 337-41
18. Venkatesan A, Benavides DR. Autoimmune encephalitis and its relation to infection. Curr Neurol Neurosci Rep. 2015. 15: 3-
19. Visvesvara GS. Infections with free-living amebae. Handb Clin Neurol. 2013. 114: 153-68