- Department of Neurosurgery, St. Marianna University School of Medicine, Toyoko Hospital, Kawasaki City, Japan
- Department of Neurosurgery, St. Marianna University School of Medicine, Kawasaki-shi, Kanagawa, Japan
Correspondence Address:
Hajime Ono
Department of Neurosurgery, St. Marianna University School of Medicine, Kawasaki-shi, Kanagawa, Japan
DOI:10.4103/2152-7806.198735
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hajime Ono, Taigen Sase, Yuichiro Tanaka, Hiroshi Takasuna. Histological assessment of porous custom-made hydroxyapatite implants 6 months and 2.5 years after cranioplasty. 19-Jan-2017;8:8
How to cite this URL: Hajime Ono, Taigen Sase, Yuichiro Tanaka, Hiroshi Takasuna. Histological assessment of porous custom-made hydroxyapatite implants 6 months and 2.5 years after cranioplasty. 19-Jan-2017;8:8. Available from: http://surgicalneurologyint.com/surgicalint_articles/histological-assessment-of-porous-custom-made-hydroxyapatite-implants-6-months-and-2-5-years-after-cranioplasty/
Abstract
Background:In cranial reconstruction, the features of artificial bone differ. Custom-made porous hydroxyapatite (HAp) implants for cranioplasty have been used all over the world because of their good cosmetic, biocompatibility, and osteoconductive properties. Surgical techniques were analyzed, and histological assessment of new bone formation in the hydroxyapatite was performed.
Methods:Over a 6-year time period, 41 patients underwent cranioplasty using a custom-made three-dimensional hybrid pore structured hydroxyapatite (3DHPoHAp) implant. The surgical techniques and histological evaluations of 3DHPoHAp in 2 cases, removed 6 months and 2.5 years after cranioplasty, are described.
Results:Using 3DHPoHAp, cranioplasty was successfully performed for all patients. The implant fit the bone defect exactly, and surgical manoeuvres were simple and easy. All implants were firmly fixed using a titanium plate, and postoperative infection occurred in 1 patient (2.4%). New bone formation was seen in 2 cases 6 months and 2.5 years after cranioplasty. Osteoblasts were progressing to the stoma at various depths, and bone tissue had ripened. Furthermore, lamellar structure was observed in the case at 2.5 years.
Conclusions:In this study, there was a low infection rate, and new bone formation was seen in vivo after cranioplasty. This study also demonstrated that the 3DHPoHAp implant is a good candidate for cranial bone implants because its good osteoconductivity and biocompatibility.
Keywords: Cranioplasty, hydroxyapatite, osteoconductivity, osteointegration, traceability
INTRODUCTION
Selection of artificial bone, including autologous bone, and reconstruction of cranial bone for cranioplasty have been entrusted to each medical institution.[
MATERIALS AND METHODS
From June 2009 to December 2014, 41 cranioplasty procedures were performed at our institution using 3DHPoHAp. The bone defects were divided into three categories by size, namely, small-sized category, including defects <150 cm2 (2 cases); medium-sized category, including defects 150–350 cm2 (18 cases); and large-sized category, including defects >350 cm2 (21 cases). This study was approved by the local ethics committee (approval no. 3368); patient consent was not required because this was a retrospective study.
Patients’ ages ranged from 31 to 85 years (mean, 65.3 years) with no sex predominance. The average period until cranioplasty was 34.8 days. There were no spontaneous fractures, rejections, or reabsorption. The features of the cases are summarized in
Design of artificial bone and the surgical technique in our institute
In the present study, the design of the artificial bone and the surgical techniques were as follows: Confirmation and antibiotic selection for systemic infection; setting the size and shape of the artificial bone using 3DCT; sufficient exposure of the autologous bone edge; cleaning the operative field and foreign body removal, including artificial dura mater; and fixation using a titanium plate and screw. Finally, in principle, antibiotics for postoperative infection control were not used except during surgery.
Manufacturing process of 3DHPoHAp
An aqueous solution of phosphoric acid was poured into a suspension of purified calcium hydroxide, which resulted in HAp slurry. The slurry was sprayed and dried using the spray-dry method to produce a fine spheroid powder. HAp powder was homogenized in water, and a water soluble polymer was added to the slurry as a binder. The slurry was gel-formed and dried. The green ceramic was shaped into a specific shape by a milling machine and then sintered at 1200°C in air. The water soluble polymer included in the green ceramic was removed by the sintering process, and pores were formed. Porosity, pore structure, and interconnecting pore size were controlled by the synthetic conditions, grain size, sintering temperature, and mixed polymer. It has been reported that APACERAM® (HOYA Technosurgical Corporation, Tokyo, Japan) has a suitable pore structure for bone regeneration and mechanical strength when these parameters are controlled.[
The 3DHPoHAp used in this study was an artificially synthesized ceramic with 40% porosity, which is currently the most commonly used cranial plate reconstruction material in Japan. This type of custom-made HAp was created by HOYA Technosurgical Corporation in 2004.
Access and cost of 3DHPoHAp
The use of custom-made HAp in Japan is not special, with each institution deciding its use. Information on 3DCT and the opinion of the neurosurgeon are important for artificial bone preparation, and in particular, the shape, curvature, and the fixed part of the artificial bone are determined. The 3DHPoHAp setting period up to cranioplasty depends on the size, however, it can be created in approximately 14 days from order. Finally, the 3DHPoHAp is sterilized packed with high-pressure steam sterilization method according to the domestic regulations in Japan. Then, it is delivered to the medical institution on the day before the surgery.
Regarding the cost of artificial bone in Japan, all artificial bones including custom-made HAp are set at a fixed price as medical equipment. The cost for artificial bone is determined by the total amount paid based on the size of the artificial bone, the patient's private income, medical cost, etc., as determined by the insurance plan. The cost of 3DHPoHAp paid by the patient is approximately $180 to $800.
RESULTS
Illustrative cases
Case 1
A 39-year-old man suffered a massive ventricular hemorrhage with immediate coma. The day after admission, he was diagnosed with Moya Moya disease by brain angiography. Although he underwent ventricular drainage of the intraventricular haemorrhage, 1 week after admission, brain herniation developed due to cerebral infarction. He then underwent immediate decompressive craniectomy using artificial dura, and intracranial pressure was controlled. After decompressive surgery, his consciousness recovered gradually. He then underwent cranioplasty using a 3DHPoHAp implant. After cranioplasty, he significantly improved by rehabilitation treatment after hospital discharge.
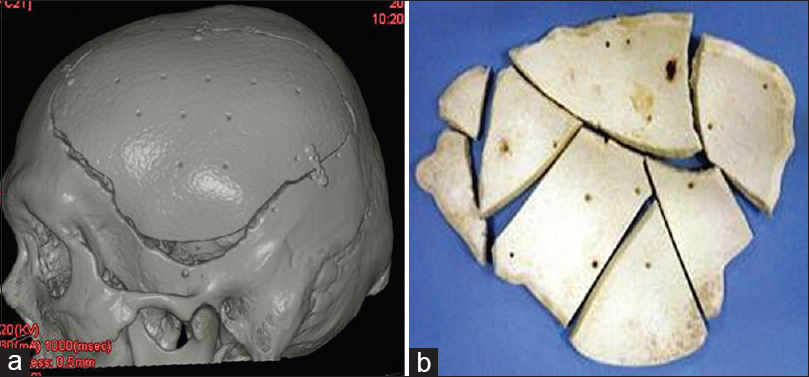
Six months after prosthesis implantation, left encephalo-myo-synangiosis was performed to improve cerebral blood flow to the Moya Moya disease. It was found that the 3DHPoHAP plate had fused tightly to the cranium and could be excised in parts using a drill and luer [
Histological findings of Case 1
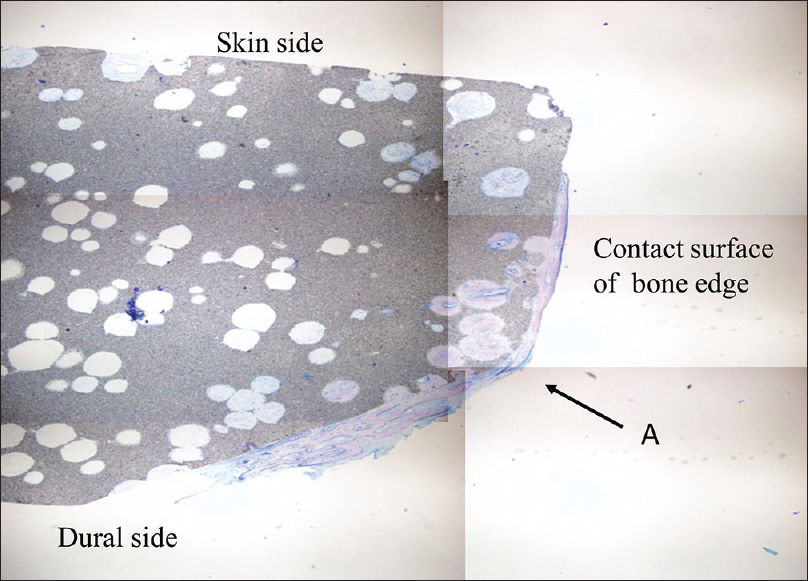
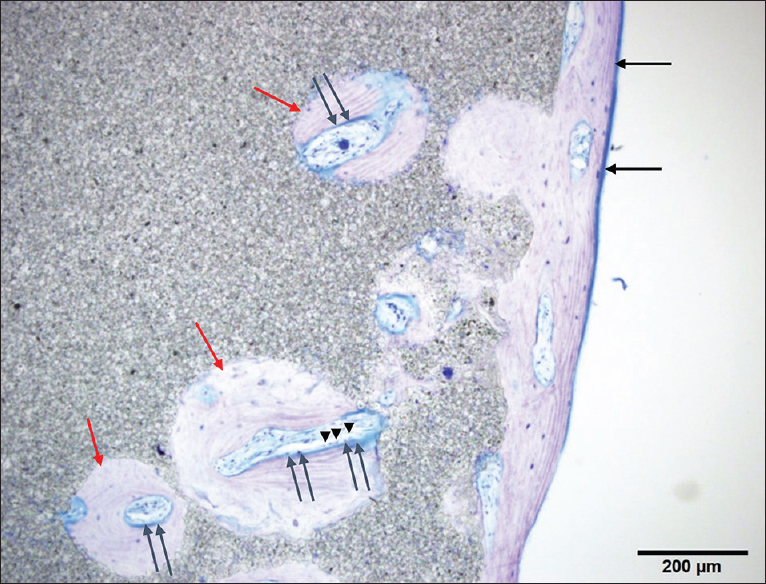
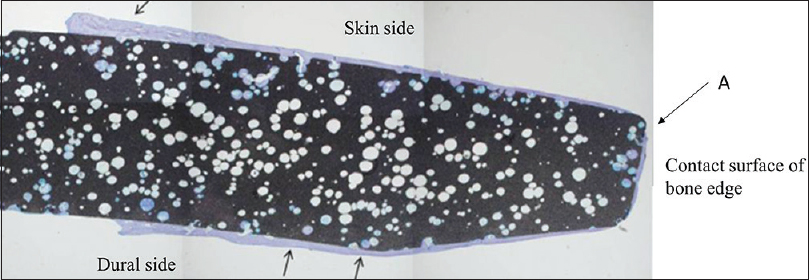
The cranial implant was fixed in 10% neutral formalin and dehydrated in alcohol. Sections approximately 400-µm thick were cut using a crystal cutter (Marutoh Co., Tokyo, Japan), ground to a thin section of approximately 50 µm, and stained with hematoxylin-eosin stain and toluidine blue. Light microscopy showed that the new bone was formed not only in the margin of the implant in direct contact with the skull but also in non-contact areas such as the implant vault or inside the pores. New bone was formed mainly parallel to the implant on the surface and in the perpendicular direction along the pores. Overall, the formation was three-dimensional. Bone formation on the dural side of the hydroxyapatite plate was more extensive than on the skin side [
Figure 3
Histological view of point A in
Case 2
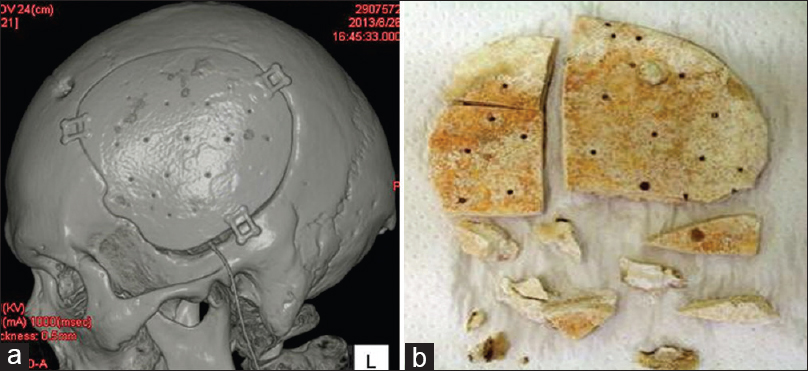
A 49-year-old man was transferred to our hospital after emergency surgical treatment with decompression for putaminal haemorrhage in a foreign medical institution. On admission to our hospital, he had meningitis due to postoperative complications, along with hydrocephalus due to the infection. After treatment of the meningitis and hydrocephalus was performed in our hospital, he eventually improved and was able to uneventfully undergo cranioplasty by 3DHPoHAp. However, postoperatively, 3DHPoHAp was removed from the surgical wound defect because of his own scratching 2.5 years later. Therefore, because the artificial bone was exposed, it was removed to avoid infection. The hydroxyapatite plate had fused tightly to the cranium without artificial dura and could be excised using a drill and luer during the procedure. It was excised in pieces in the same manner as in Case 1 [
Histological findings of Case 2
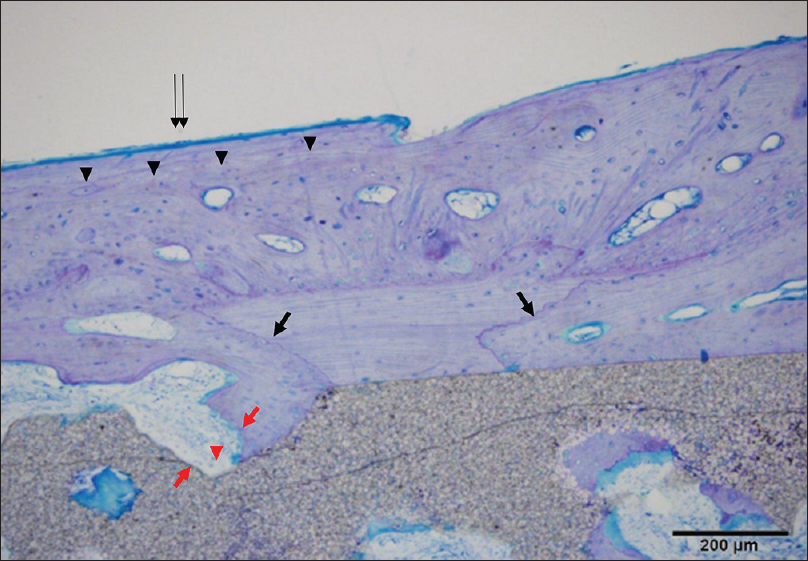
Histological sections were processed in the same manner as in Case 1. New bone formation was more mature than that seen after 6 months in the pores of the plate between the cranium and implant. Light microscopy showed that new bone was formed mainly parallel to the implant on the surface and in the perpendicular direction along the pores. The artificial bone tissue layer plate structure was observed. Scalloping that was absorbed into mature bone with a lamellar structure on the surface of the new bone was recognized as osteoid, suggesting thriving bone formation. Artificial bone and bone tissue boundaries in the vicinity of the absorption fossa and osteoclast-like tissue were observed [Figures
Figure 6
Histological view of point A in
DISCUSSION
The purpose of this study was to histologically evaluate the new bone formation of HAp implants. It has previously been shown that a prosthesis made of a HAp implant induces new bone formation in experimental animal models. According to previous reports of histological investigations, HAp implants demonstrated osteointegration in animal model studies.[
In a bone healing situation, such as a fracture, the majority of bone healing is dependent on osteoinduction.[
Also related to intensity, it is important to have a biocompatible material with suitable porosity in a HAp implant. Then, an appropriate spatial distribution of suitable holes with the correct size and shape to allow migration of osteoblasts from the living bone to occur results in osteointegration.
Therefore, these properties of implants will lead to the biocompatibility of the artificial bone. HAp implants have generally been noted to have many advantages in some past reports.[
However, in comparison with other artificial bone, there is not much evidence for osteointegration of HAp implants for humans in vivo.[
Including the porosity of the implant, Marco et al. demonstrated new bone formation with a HAp implant after cranioplasty. They histologically evaluated three different porosities of the implants used in two cases. The porosities of the three different implants were 48.8%, 42.9%, and 55.8%. With the implant with a porosity of 42.9%, there was no new bone formation 6 months after cranioplasty. As one of the reasons for this, they noted shortened period of cranioplasty and explantation.[
In the present cases, new bone formation was observed at 6 months and 2.5 years after cranioplasty using 3DHPoHAP implants because the implant had porous characteristics and there was binding of the artificial bone and the marginal cranium edge. The implant used was formed of a 3D porous body with various pore sizes, taking into account new bone formation. Therefore, the porosity of the implants was 40%, with 5-mm thickness to provide sufficient strength.[
In the perioperative period, the implant was designed by 3DCT, and the boundary between the artificial bone and the marginal cranium edge was as small as possible, which significantly increases the contact surface of both. As for the operative technique, the remaining connective tissue on the marginal cranium edge and the epidural membrane were removed and washed sufficiently. The small titanium plates were fixed by screws.
Regarding the timing of cranioplasty after decompression, there are some opinions that 2–6 months to cranioplasty are required to avoid postoperative infectious complications;[
When comparing the present approach to the use of autologous bone, the problems with the autologous bone use are the complicated preservation method and deformation, and it is clear that there is a particular risk of infection, as well as with artificial bone. Of course, autogenous bone is ideal for cranioplasty. However, because there are not a few infections of autologous bone,[
Furthermore, due to the vulnerability of HAp implants, it has been reported that they cannot be used for large bone defects. However, there is no problem for a fixed defect, providing brain protection for activities of daily living of the patients in the present study. Therefore, the strength of Hap implants for large defects needs to be examined in the future.
This research has some limitations. Because only Hap implants were used in cranioplasty, comparisons with other artificial bones, including other porous hand-made Hap implants, were not possible. When using HAp implants, a long period of observation is needed, and there is a need to consider several issues in the future. With respect to HAp implants, there is no correct answer for selecting artificial bone. However, for artificial bone, which is created in the same manner, to be used, a system of product traceability is needed.
Finally, the main issue is that HAp implants are manufactured in different ways in each country. Therefore, constant evaluation of postoperative outcomes is difficult. Thus, information about artificial bone will become very important in the future. Neurosurgeons must understand the product to demonstrate its effectiveness, and they must cooperate in sharing information to improve management of patients treated with artificial bone implants.[
CONCLUSIONS
The present histological study of 2 3DHPoHAp plates removed after use for 6 months and 2.5 years, respectively, showed fusion of the 3DHPoHAp to the cranium and new bone formation, mainly on the dural side and in a 3D matrix within pores. 3DHPoHAp showed osteointegration and lamellar organization in the two cases presented. The 3DHPoHAp implant appears to be an excellent candidate as a cranial bone substitute due to its osteoconductivity and biocompatibility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Mr. Takehiko Nakajima and Mr. Hirofumi Matsuzawa (HOYA Technosurgical Corporation, Tokyo, Japan) for their advice.
References
1. Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007. 28: 4240-50
2. Costantino PD, Hiltzik D, Govindaraj S, Moche J. Bone healing and bone substitutes. Facial Plast Surg. 2002. 18: 13-26
3. Flautre B, Descamps M, Delecourt C, Blary MC, Hardouin P. Porous HA ceramic for bone replacement: Role of the pores and interconnections-experimental study in the rabbit. J Mater Sci Mater Med. 2001. 12: 679-82
4. Frassanito P, De Bonis P, Mattogno PP, Mangiola A, Novello M, Brinchi D. The fate of a macroporous hydroxyapatite cranioplasty four years after implantation: Macroscopical and microscopical findings in a case of recurrent atypical meningioma. Clin Neurol Neurosurg. 2013. 115: 1496-8
5. Fricia M, Passanisi M, Salamanna F, Parrilli A, Giavaresi G, Fini M. Osteointegration in custom-made porous hydroxyapatite cranial implants: From reconstructive surgery to regenerative medicine. World Neurosurg. 2015. 84: 591.e11-6
6. Hobar PC, Masson JA, Wilson R, Zerwekh J. The importance of the dura in craniofacial surgery. Plast Reconstr Surg. 1996. 98: 217-25
7. Hng D, Bhaskar I, Khan M, Budgeon C, Damodaran O, Knuckey N. Delayed cranioplasty: Outcomes using frozen autologous bone flaps. Craniomaxillofac Trauma Reconstr. 2015. 8: 190-7
8. Itokawa H, Hiraide T, Moriya M, Fujimoto M, Nagashima G, Suzuki R. A 12 month in vivo study on the response of bone to a hydroxyapatite-polymethylmethacrylate cranioplasty composite. Biomaterials. 2007. 28: 4922-7
9. Kawamoto H. Experimental studies on mandibular reconstruction porous hydroxyapatite ceramics. Medical journal of Kobe University. 1991. 52: 109-19
10. Marcacci M, Kon E, Zaffagnini S, Giardino R, Rocca M, Corsi A. Reconstruction of extensive long-bone defects in sheep using porous hydroxyapatite sponges. Calcif Tissue Int. 1999. 64: 83-90
11. Martini L, Staffa G, Giavaresi G, Salamanna F, Parrilli A, Serchi E. Long-term results following cranial hydroxyapatite prosthesis implantation in a large skull defect model. Plast Reconstr Surg. 2012. 129: e625-35
12. Messina G, Dones I, Nataloni A, Franzini A. Histologically demonstrated skull bone integration in a hydroxyapatite prosthesis in a human. Acta Neurochir. 2011. 153: 1717-8
13. Okii N, Nishimura S, Kurisu K, Takeshima Y, Uozumi T. In vivo histological changes occurring in hydroxyapatite cranial reconstruction-case report. Neurol Med Chir. 2001. 41: 100-4
14. Ono I, Tateshita T, Nakajima T. Porous hydroxyapatite ceramics and their ability to be fixed by commercially available screws. Biomaterials. 1999. 20: 1595-602
15. Ono I, Tateshita T, Nakajima T, Ogawa T. Determinations of strength of synthetic hydroxyapatite ceramic implants. Plast Reconstr Surg. 1998. 102: 807-13
16. Ono I, Tateshita T, Satou M, Sasaki T, Matsumoto M, Kodama N. Treatment of large complex cranial bone defects by using hydroxyapatite ceramic implants. Plast Reconstr Surg. 1999. 104: 339-49
17. Piedra MP, Ragel BT, Dogan A, Coppa ND, Delashaw JB. Timing of cranioplasty after decompressive craniectomy for ischemic or hemorrhagic stroke. J Neurosurg. 2013. 118: 109-14
18. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V. Cranioplasty after decompressive craniectomy: The effect of timing on postoperative complications. J Neurotrauma. 2012. 29: 1090-5
19. Shah AM, Jung H, Skirboll S. Material used in cranioplasty: A history and analysis. Neurosug Focus. 2014. 36: E19-
20. Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R. Custom made bioceramic implants in complex and large cranial reconstruction: A two-year follow-up. J Craniomaxillofac Surg. 2012. 40: e65-70
21. Staffa G, Nataloni A, and Compagnone C, Servadei F. Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir. 2007. 149: 161-70
22. Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. Cranial bone defects: Current and future strategies. Neurosurg Focus. 2010. 29: E8-
23. Tanaka Y. Development of titanium fixation screw for hydroxyapatite osteosynthesis (APACERAM®). Surg Neurol. 2008. 70: 545-9
24. Teixeira S, Fernandes H, Leusink A, van Blitterswijk C, Ferraz MP, Monteiro FJ. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res A. 2010. 93: 567-75
25. Verheggen R, Merten HA. Correction of skull defects using hydroxyapatite cement (HAC): Evidence derived from animal experiments and clinical experience. Acta Neurochir. 2001. 143: 919-26
26. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011. 68: 1124-9
27. Zanotti B, Verlicchi A, Indiani S, Scarparo SA, Zingaretti N, Parodi PC. Spontaneous fractures in custom-made porous hydroxyapatite cranioplasty implants: Is fragility the only culprit?. Acta Neurochir. 2015. 157: 517-23







C. David Hunt
Posted March 6, 2017, 6:27 pm
This small jewel of a paper beautifully demonstrates two
things. First, and most specifically, it demonstrates that
any topic, when examined carefully from the correct
perspective, can add precise and useful information to
our body of knowledge. In this case, the characterization
of the hydroxyappetite implants, and their relationship
to successful incorporation, is well documented and
insightful.
Second, it demonstrated the ability of physician–corporate
partnerships to better care for patients, in a regulatory
environment which assures both adequate corporate
profit and assured patient access.