- Department of Neurosurgery, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Neurosurgery and Internal Medicine, Division of Neurology, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Internal Medicine, Division of Neurology, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Radiology, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Psychiatry, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Radiology, Division of Neuroradiology, Hackensack University Medical Center, Hackensack, NJ, USA
- Department of Anesthesiology, Hackensack University Medical Center, Hackensack, NJ, USA
Correspondence Address:
Hooman Azmi
Department of Anesthesiology, Hackensack University Medical Center, Hackensack, NJ, USA
DOI:10.4103/2152-7806.187537
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Azmi H, Gupta F, Vukic M, Kreitner J, Kera E, Nicola G, Pierce S, Panush D, Cohen R. Interventional magnetic resonance imaging-guided subthalamic nucleus deep brain stimulation for Parkinson's disease: Patient selection. Surg Neurol Int 02-Aug-2016;7:
How to cite this URL: Azmi H, Gupta F, Vukic M, Kreitner J, Kera E, Nicola G, Pierce S, Panush D, Cohen R. Interventional magnetic resonance imaging-guided subthalamic nucleus deep brain stimulation for Parkinson's disease: Patient selection. Surg Neurol Int 02-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/interventional-magnetic-resonance-imaging%e2%80%91guided-subthalamic-nucleus-deep-brain-stimulation-parkinsons-disease-patient-selection/
Abstract
Background:Interventional magnetic resonance imaging (iMRI) guided deep brain stimulation (DBS) for Parkinson's disease (PD) has been shown to be effective. The costs of a dedicated intraoperative MRI may be prohibitive. The procedure can also be performed in a diagnostic scanner, however this presents challenges for utilization of time when the scanner is used both as a diagnostic and an interventional unit. This report outlines our novel methodology for patient selection for implantation in a diagnostic MR scanner, as an attempt to streamline the use of resources. A retrospective review of our outcomes is also presented.
Methods:DBS candidacy evaluation included a PD questionnaire-39. Anxiety, age, difficulties in communication and body habitus were factors that were assessed in selecting patients for this technique. Eleven patients underwent iMRI-guided DBS implantation in the subthalamic nucleus. All patients were implanted bilaterally. Unified PD rating scale (UPDRS) part III and L-dopa dose were compared pre- and post-stimulation. A cohort of 11 DBS patients not selected for iMRI-guided DBS were also reported for comparison.
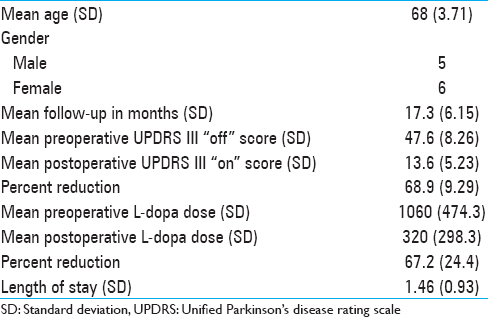
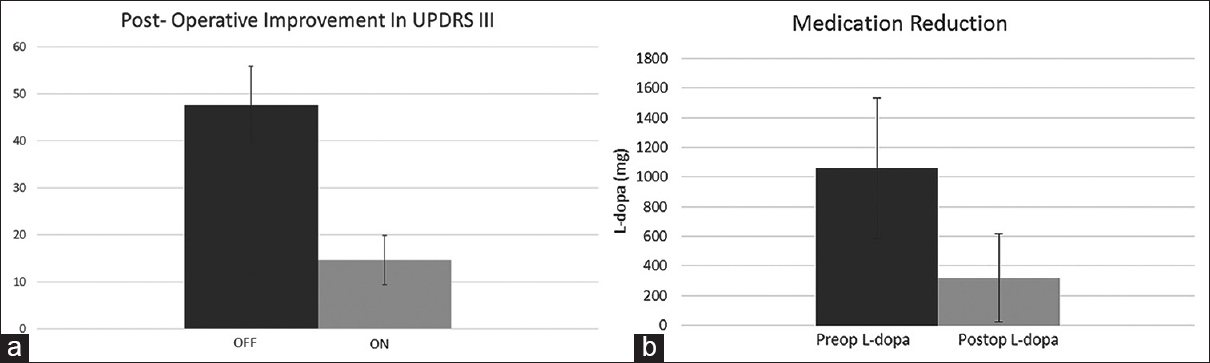
Results:For the iMRI-guided patients, mean “Off” UPDRS III score was 47.6 (standard deviation [SD] 8.26). Postoperative “On” medication, “On” stimulation UPDRS III was 13.6 (SD 5.23). Mean preoperative L-dopa dose was 1060 mg (SD 474.3) and mean postoperative L-dopa dose was 320 (SD 298.3).
Conclusion:iMRI-guided DBS is a newly emerging technique for surgical treatment of patients with PD. We present a novel scoring system for patient selection assessing anxiety, age, ability to communicate, and body habitus to identify patients who will be benefited most from this technique.
Keywords: Anesthesia, deep brain stimulation, magnetic resonance imaging-guided deep brain stimulation, Parkinson's disease, patient selection
INTRODUCTION
Deep brain stimulation (DBS) has become a well-established surgical treatment option for medically refractory Parkinson's disease (PD).[
The implantation of the DBS electrode with these means requires the patient to be kept awake during parts of the surgery, and for PD medications to be held. While this approach has been advanced across centers to ensure comfort and safety, there are still groups of patients for whom this may be difficult. DBS lead placement requiring patient interaction may present a challenge for those with significant anxiety, those who are older, those with communication difficulty from Parkinsonian symptoms or language barriers, or patients in whom airway control may be a concern.
Recently, Starr et al. have described an interventional magnetic resonance imaging (iMRI) guided method for DBS implantation which allows the use of general anesthesia with good results.[
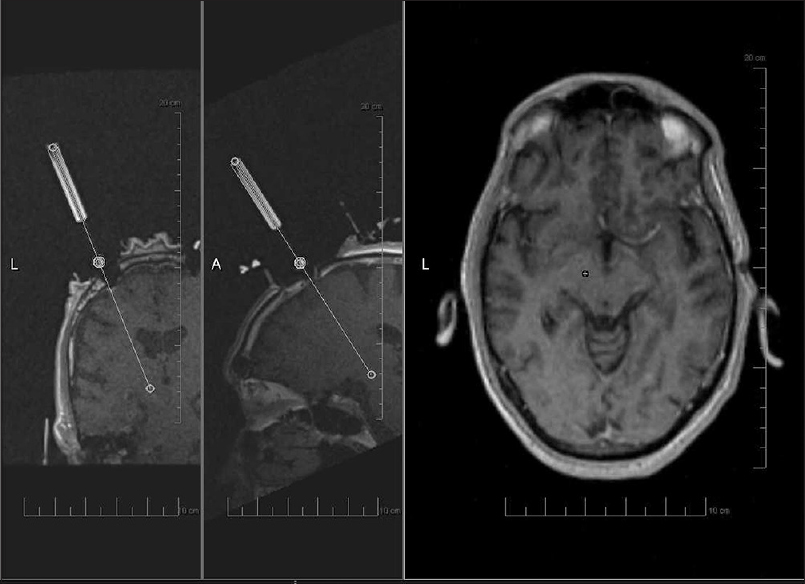
Figure 1
Image acquired during planning for interventional magnetic resonance imaging-guided deep brain stimulation with ClearPoint technique. From left to right, panels show coronal and sagittal images of guide cannula being aligned with planned trajectory. Axial image showing planned target (circle) correlating with actual trajectory (plus sign)
This manuscript outlines our methodology for selecting patients for DBS implantation in the subthalamic nucleus (STN) using the ClearPoint system with a diagnostic scanner, with the aim of most efficient use of MRI time. We retrospectively report our outcomes for these patients. For comparison, outcomes of a cohort of DBS patients who underwent conventional MER-guided implantation are also reported.
METHODS
All patients being assessed for DBS candidacy underwent a dopamine challenge test, a neuropsychological evaluation, and a contrast-enhanced MRI of the brain. All patients also completed the PD questionnaire (PDQ)-39 preoperatively.[
Eleven patients were selected in this manner and underwent iMRI-guided STN DBS implantation between September 2011 and April 2013. The demographics of this group are demonstrated in
All iMRI-guided implantations were performed under general anesthesia. An excellent description of the iMRI technique has been described in detail elsewhere.[
We used a 1.5T diagnostic Siemens Symphony scanner (Siemens Medical Solutions, Malvern, PA, USA) for the procedures. The scanner is located in a standard MRI suite, in the radiology facilities, and some changes were made to the overhead ventilator system prior to initiating the program.
All eleven patients underwent simultaneous bilateral STN implantation with Medtronic 3389 DBS electrodes (Minneapolis, MN, USA). Parkinson's medications were not stopped prior to surgery and were continued postoperatively. A noncontrast-enhanced high-resolution computed tomography (CT) scan was obtained postoperatively in all patients for lead confirmation and for surveillance of hemorrhage. The mean follow-up period has been 17.3 months (SD 6.15). For MER-guided DBS, we used a Cosman Roberts Wells stereotactic frame (CRW) (Integra Life Sciences, Plainsboro, NJ, USA) stereotactic frame and utilized CT and MRI fusion for surgical planning, and an Alpha-Omega recording system (Alpha Omega Company, USA Inc, Alpharetta, GA, USA) for neurophysiological assessment. All 11 patients in this cohort also underwent simultaneous bilateral STN implantation with Medtronic 3389 DBS electrodes.
For evaluation of patients, Unified PD rating scale (UPDRS) ratings were obtained by a single un-blinded rater, and recorded at each patient visit. Baseline UPDRS “Off” scores were obtained on the first programing visit after withholding PD medications overnight. The reported “On” scores reflect the UPDRS part III with patients “On” stimulation and “On” medications at the most recent postoperative visit. A preoperative dopamine challenge test was also performed on all patients. PD medications were withheld at least 12 h prior to the evaluation, a UPDRS part III score was obtained and again repeated after administrations of medications.
RESULTS
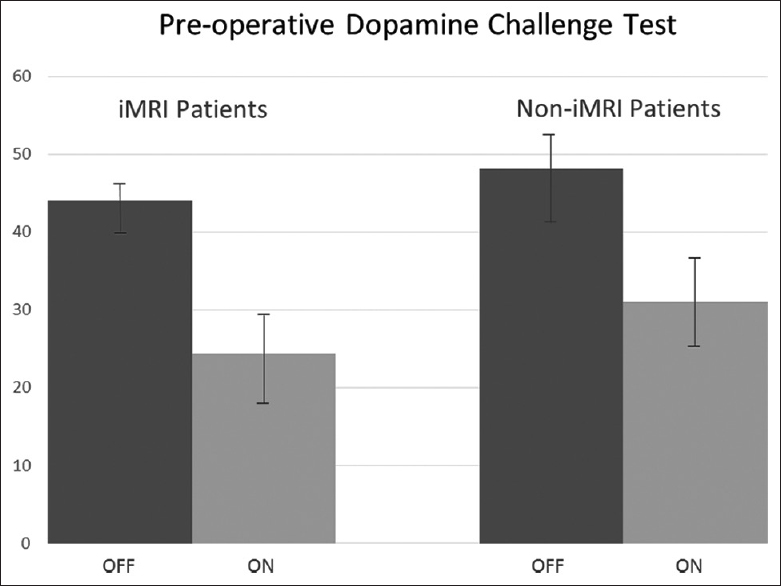
A retrospective review of the patients was conducted upon approval from the institutional review board. The iMRI-guided cohort had a mean “Off” score of 43.9 (SD 2.9) and a mean “On” score of 24.3 (SD 5.9) for the preoperative dopamine challenge test, and the non-iMRI guided cohort had a mean “Off” score of 48.1 (SD 5.1) and a mean “On” score of 31.1 (SD 6.3) for an improvement of 44.1% (SD 13.9) and 35.5% (SD 8.8), respectively, and this did not reach significance (P > 0.11) [
Figure 2
Preoperative dopamine challenge test in interventional magnetic resonance imaging-guided cohort and noninterventional magnetic resonance imaging-guided cohort. “Off” Unified Parkinson's disease rating scale III score (dark gray) compared to “On” Unified Parkinson's disease rating scale score (light gray)
Figure 3
(a) Improvement in Unified Parkinson's disease rating scale III in iMRI-guided cohort. Graph showing “Off” Unified Parkinson's disease rating scale III score (dark gray) compared to postoperative “On” Unified Parkinson's disease rating scale III score (light gray). (b) Medication reduction: Graph showing preoperative L-dopa dose (dark gray) compared to postoperative L-dopa dose (light gray) in iMRI-guided cohort
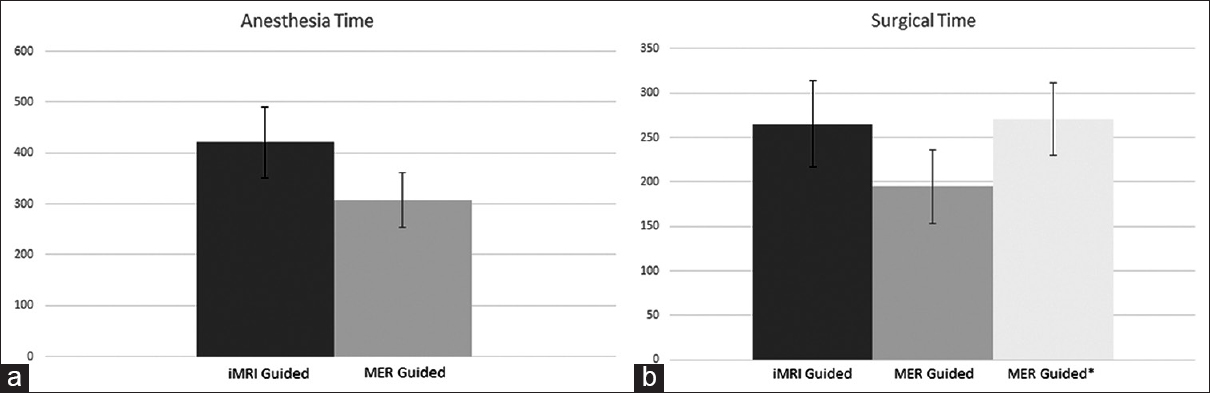
The mean anesthesia time for the MRI-guided patients was 420.9 min (SD 69.3) and the mean surgical time was 265 min (SD 48.4). In comparison, the MER-guided cohort had a mean time of 308 min (SD 54.4) for anesthesia and 195 (SD 41.1) surgical time. This difference in the two groups was statistically significant for both anesthesia time (P < 0.0007) and for surgical times (P < 0.003). For the MER-guided cohort, the mean time from stereotactic frame placement to end of surgery was 270.9 min (SD 41.1) [
Figure 5
(a) Anesthesia time: Graph showing anesthesia time in minutes for interventional magnetic resonance imaging-guided cohort (dark gray) compared to microelectrode recording guided cohort (light gray). (b) Surgical time: Graph showing surgical times in minutes for interventional magnetic resonance imaging-guided cohort (dark gray), and microelectrode recording guided cohort from skin incision to closure (light gray) and from placement of stereotactic frame to closure (lighter gray with asterisk)
There were no hemorrhagic or infectious complications in either cohort, and the length of stay for the MRI cohort was 1.46 days and for the MER cohort, it was 1 day.
DISCUSSION
Recently, iMRI-guided DBS placement has been shown to have outcomes comparable with MER-guided implantation. The hesitation to forego intra-operative MER and stimulation assessment, as well as the logistics and the costs in the installation of dedicated intra-operative MRIs represents obstacles to the adoption of this procedure. An alternative to the expense of a dedicated intra-operative scanner is the use of a diagnostic iMRI. This, however, may present challenges in allocation of resources, most importantly, the time allotted on the diagnostic scanner.
At our institution, we have implemented this technique using a 1.5T diagnostic scanner. We are currently considering this technique for a selected group of patients who while deemed good surgical candidates, may present challenges to the team in traditional MER-guided surgery. While we would like to offer the technique to more patients, we are prohibited by the logistics of time allotment on a diagnostic scanner, and our selection process has been designed to offer the technique to patients who need it most, and at the same time, appropriate the use of the scanner efficiently.
For patients deemed good candidates for surgery, the multidisciplinary team considers four factors for selection of the surgical technique. We have found a composite scale which takes into consideration the patients’ degree of anxiety, their age, their body habitus, and their ability to communicate with the surgical team during the implantation to be most valuable in the selection process. The scoring system is summarized in
Anxiety
Anxiety is commonly observed in patients with PD.[
Communication
The ability to select the best target for implantation during MER-guided surgery depends partly on reliably communicating with patients during surgery. While interpreters are provided in the operating room for non-English speaking patients for MER-guided implantation, there are concerns for subtle points being “lost in translation.” Moreover, regardless of their native language, there are those patients whose ability to communicate is affected by PD, unnecessarily contributing to difficulties in surgery, a possible unpleasant experience or worse, compromise of safety. For this group also, we have found the iMRI technique to offer an advantage over MER-guided implantation.
Age
While age is currently not considered a limiting factor in DBS surgery for PD, older patients may have more difficulty during MER-guided surgery. Medication withdrawal may be less tolerated in these patients, communication may be more difficult for them, and in general, instructions may be more difficult for them to follow. These all may contribute to more challenges in performing MER-guided surgery. iMRI-guided DBS placement seems to offer an advantage for this group of patients as well.
Body habitus
Airway obstruction during sedation may occur for some patients. A patient's body habitus and cervical anatomy may amplify this issue [
While the main purpose of this study is not the demonstration of the efficacy of iMRI-guided DBS, we are reporting our results. Our method of reporting these outcomes may be a cause for criticism. The postoperative improvement in DBS is generally measured by assessing the effects of stimulation with patients being “Off” medication. This “Off” medication “On” stimulation measure of UPDRS part III is the standard primary outcome measure for studies which report the efficacy of DBS and has been reported in the range of 41–54%.[
This manuscript is an attempt to describe a selection process, and we have also retrospectively reported our outcomes. Other authors have described the efficacy of this technique in a larger group of patients. While our outcomes seem in line with these larger studies, one of the shortcomings of this paper is the small size. Clearly, more patients will be needed to draw any conclusions.
A potential shorter operating time is one of the advantages that make iMRI-guided DBS surgery an attractive option. Our surgical and anesthesia times were slightly higher in this iMRI-guided cohort [
Another criticism may be the use of the PDQ-39 for the assessment of anxiety instead of other scales. While anxiety scales may be more comprehensive, none have been developed specifically for Parkinson's patients,[
In addition, consideration should be given to the potential risks of prolonged general anesthesia in the Parkinson's population. While we did not experience any anesthesia-related complications, there may be respiratory and cardiovascular effects of anesthesia, specific to patients with PD.[
CONCLUSION
MRI-guided DBS implantation offers an excellent method for DBS implantation under general anesthesia. While greatly improving patient anxiety and comfort in the operating room, its use, especially in centers that perform this procedure in the diagnostic suite may present challenges for resource allocation. We have identified a selection criterion using a composite scale that allows us to offer this technique to patients who would benefit most, while being sensitive to utilization of time on the MRI scanner. This is a small series and clearly more patients will be needed to draw any significant conclusions. Nevertheless, we have found the selection process to be a useful tool in identifying the patients for either technique, we have also found the outcomes between the two methods to be fairly similar, and both techniques had equally low complications rates.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Hooman Azmi: Consulting agreement with Medtronic Inc.
Fiona Gupta: Consulting agreement with Medtronic Inc., Teva Pharmaceuticals, UCB Pharmaceuticals, US Worldmeds, Allergan Pharmaceuticals.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Michael Schulder for his invaluable editorial comments.
References
1. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006. 355: 896-908
2. Dissanayaka NN, Sellbach A, Matheson S, O'sullivan JD, Silburn PA, Byrne GJ. Anxiety disorders in Parkinson's disease: Prevalence and risk factors. Mov Disord. 2010. 25: 838-45
3. Forjaz MJ, Martinez-Martin P, Dujardin K, Marsh L, Richard IH, Starkstein SE. Rasch analysis of anxiety scales in Parkinson's disease. J Psychosom Res. 2013. 74: 414-9
4. Larson PS, Starr PA, Bates G, Tansey L, Richardson RM, Martin AJ. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: Preliminary evaluation of targeting accuracy. Neurosurgery. 2012. 70: 95-103
5. Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Anxiety rating scales in Parkinson's disease: A validation study of the Hamilton anxiety rating scale, the beck anxiety inventory, and the hospital anxiety and depression scale. Mov Disord. 2011. 26: 407-15
6. Nicholson G, Pereira AC, Hall GM. Parkinson's disease and anaesthesia. Br J Anaesth. 2002. 89: 904-16
7. Ondo WG, Bronte-Stewart H. The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg. 2005. 83: 142-7
8. Ostrem JL, Galifianakis NB, Markun LC, Grace JK, Martin AJ, Starr PA. Clinical outcomes of PD patients having bilateral STN DBS using high-field interventional MR-imaging for lead placement. Clin Neurol Neurosurg. 2013. 115: 708-12
9. Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: A review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998. 245: S10-4
10. Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SA, Grill S. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson's disease. Mov Disord. 2009. 24: 1333-8
11. Rezai AR, Machado AG, Deogaonkar M, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurgery. 2008. 62: 809-38
12. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S. Bilateral deep brain stimulation in Parkinson's disease: A multicentre study with 4 years follow-up. Brain. 2005. 128: 2240-9
13. Schüpbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM. Stimulation of the subthalamic nucleus in Parkinson's disease: A 5 year follow up. J Neurol Neurosurg Psychiatry. 2005. 76: 1640-4
14. Starr PA, Martin AJ, Larson PS. Implantation of deep brain stimulator electrodes using interventional MRI. Neurosurg Clin N Am. 2009. 20: 193-203
15. Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: Technique and application accuracy. J Neurosurg. 2010. 112: 479-90
16. Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA. 2009. 301: 63-73
17. Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): A randomised, open-label trial. Lancet Neurol. 2010. 9: 581-91