- Department of Neurosurgery, Baylor College of Medicine, Texas Children's Hospital, Houston, TX 77030, USA
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Baylor College of Medicine, Texas Children's Hospital, Houston, TX 77030, USA
DOI:10.4103/2152-7806.161413
Copyright: © 2015 Lam S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Lam S, Reddy GD, Lin Y, Jea A. Management of hydrocephalus in children with posterior fossa tumors. Surg Neurol Int 23-Jul-2015;6:
How to cite this URL: Lam S, Reddy GD, Lin Y, Jea A. Management of hydrocephalus in children with posterior fossa tumors. Surg Neurol Int 23-Jul-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/management-of-hydrocephalus-in-children-with-posterior-fossa-tumors/
Abstract
Keywords: Endoscopic third ventriculostomy, hydrocephalus, pediatric, posterior fossa tumor, ventriculoperitoneal shunt
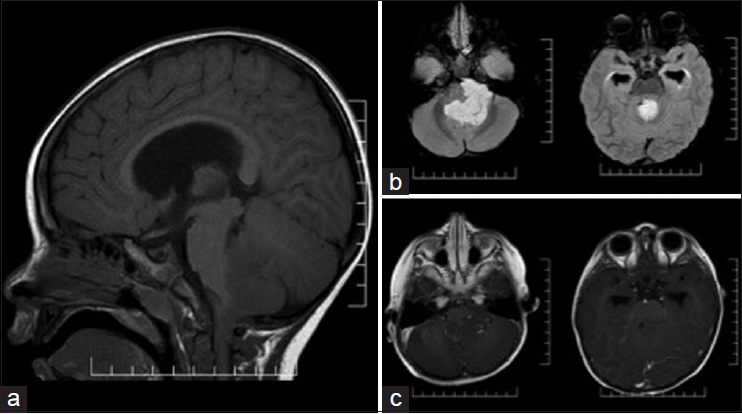
Case 1: A 2-year-old male with no prior medical history presented to the emergency room with a 3-week history of constant headache and daily vomiting. Computed tomography (CT) and subsequent magnetic resonance imaging (MRI) of the brain [
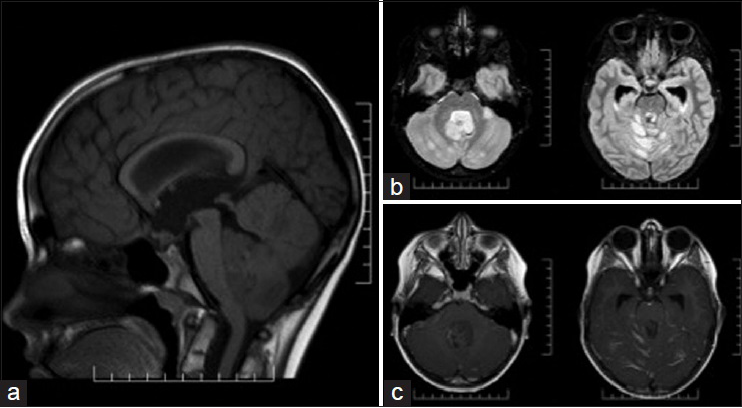
Case 2: A 9-year-old male with no prior medical history presented to an outside hospital emergency room with 2 weeks of progressive headaches and 1-day of vomiting. A CT of the head showed a posterior fossa mass. MRI of the brain [
INTRODUCTION
Central nervous system tumors are the most common solid tumors in children, and they predominantly occur in the posterior fossa.[
MANAGEMENT
The optimal management of hydrocephalus in a child with a posterior fossa tumor is a topic of debate.[
Purported benefits of permanent preresection CSF diverting surgery, such as ETV of VPS other than the reduced incidence of postresection hydrocephalus, include the following: (1) Being able to delay resection surgery, thus avoiding resection under emergent conditions or allowing for preresection adjuvant therapy in certain circumstances;[
FACTORS PREDICTIVE OF POSTRESECTION HYDROCEPHALUS
Ideally, we would be able to predict which patients will develop postresection hydrocephalus. The benefits of early CSF diversion could be captured while simultaneously avoiding the harm of subjecting patients to unnecessary procedures. Many groups have attempted to analyze retrospective data looking for clinical factors associated with a need for postoperative CSF diversion. Culley et al. (n = 117, 1976–1990) found that age <3 years, midline tumor location, subtotal resection, prolonged EVD requirement, cadaveric (vs. autologous) dural grafts, pseudomeningocele formation, and CSF infections were statistically significant factors associated with the need for postoperative shunt placement.[
In 2009, Riva-Cambrin et al. used a cohort of 343 patients to develop a clinical prediction rule for postresection hydrocephalus and validated it against another cohort of 111 patients from another institution in an attempt to identify high-risk patients who would benefit most from prophylactic ETV.[
TREATMENT RECOMMENDATIONS
There exists no class I evidence in the literature to guide the management of hydrocephalus in children with posterior fossa tumors. It is possible to draw guidance from the extant data highlighted above. As the overall incidence of postresection hydrocephalus is typically 30%, any anticipated benefit should be weighed against exposing the patient to more surgery or permanent shunt implantation. It is noted that in lower resource settings, there may be other considerations, including the cost of care, access to the operating room and need to minimize the number of surgeries. In our practice, in cases where there is no hydrocephalus on presentation, preresection CSF diversion is not done. In cases where there is symptomatic hydrocephalus on presentation, preresection EVD, VPS or ETV should be applied as clinically appropriate. EVD is favored for its advantages of expedient placement, external control over drainage perioperatively, and egress of resection-related blood and protein products. In cases where the child possesses multiple described risk factors for the development of postresection hydrocephalus, preresection prophylactic CSF diversion may be considered. Overall, close observation is recommended, with a preference for expectant management, rather than prophylactic surgery, and postresection definitive CSF diversion procedures undertaken only as clinically necessary.
References
1. Bognár L, Borgulya G, Benke P, Madarassy G. Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst. 2003. 19: 332-6
2. Culley DJ, Berger MS, Shaw D, Geyer R. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery. 1994. 34: 402-7
3. Di Rocco F, Jucá CE, Zerah M, Sainte-Rose C. Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg. 2013. 79: S18.e15-9
4. Due-Tønnessen BJ, Helseth E. Management of hydrocephalus in children with posterior fossa tumors: Role of tumor surgery. Pediatr Neurosurg. 2007. 43: 92-6
5. Foreman P, McClugage S, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS. Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. 2013. 12: 220-6
6. Fritsch MJ, Doerner L, Kienke S, Mehdorn HM. Hydrocephalus in children with posterior fossa tumors: Role of endoscopic third ventriculostomy. J Neurosurg. 2005. 103: 40-2
7. Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC. Childhood brain tumor epidemiology: A brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014. 23: 2716-36
8. Kumar V, Phipps K, Harkness W, Hayward RD. Ventriculo-peritoneal shunt requirement in children with posterior fossa tumours: An 11-year audit. Br J Neurosurg. 1996. 10: 467-70
9. Morelli D, Pirotte B, Lubansu A, Detemmerman D, Aeby A, Fricx C. Persistent hydrocephalus after early surgical management of posterior fossa tumors in children: Is routine preoperative endoscopic third ventriculostomy justified?. J Neurosurg. 2005. 103: 247-52
10. Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R. Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. 2009. 3: 378-85
11. Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M. Management of hydrocephalus in pediatric patients with posterior fossa tumors: The role of endoscopic third ventriculostomy. J Neurosurg. 2001. 95: 791-7
12. Santos de Oliveira R, Barros Jucá CE, Valera ET, Machado HR. Hydrocephalus in posterior fossa tumors in children. Are there factors that determine a need for permanent cerebrospinal fluid diversion?. Childs Nerv Syst. 2008. 24: 1397-403
13. Schijman E, Peter JC, Rekate HL, Sgouros S, Wong TT. Management of hydrocephalus in posterior fossa tumors: How, what, when?. Childs Nerv Syst. 2004. 20: 192-4