- Neurosurgical and Obstetrics Departments of Servidores do Estado Hospital, RJ, Brazil
Correspondence Address:
Jose Carlos Lynch
Neurosurgical and Obstetrics Departments of Servidores do Estado Hospital, RJ, Brazil
DOI:10.4103/2152-7806.200575
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Celestino Esteves Pereira, Jose Carlos Lynch. Management strategies for neoplastic and vascular brain lesions presenting during pregnancy: A series of 29 patients. 20-Feb-2017;8:27
How to cite this URL: Celestino Esteves Pereira, Jose Carlos Lynch. Management strategies for neoplastic and vascular brain lesions presenting during pregnancy: A series of 29 patients. 20-Feb-2017;8:27. Available from: http://surgicalneurologyint.com/surgicalint_articles/management-strategies-for-neoplastic-and-vascular-brain-lesions-presenting-during-pregnancy-a-series-of-29-patients/
Abstract
Background:The occurrence of a brain tumor or intracranial vascular lesion during pregnancy is a rare event, but when it happens, it jeopardizes the lives of both the mother and infant. It also creates challenges of a neurosurgical, obstetric, and ethical nature. A multidisciplinary approach should be used for their care.
Methods:Between 1986 and 2015, 12 pregnant women diagnosed with brain tumors and 17 women with intracranial vascular lesion underwent treatment at the Neurosurgery Department of the Servidores do Estado Hospital and Rede D’Or/São Luis. The Neurosurgery Department teamed up with Obstetrics Anesthesiology Departments in establishing the procedures. The patients’ records, surgical descriptions, imaging studies, and histopathological material were reviewed.
Results:Among 12 patients presenting with brain tumors, there were neither operative mortality nor fetal deaths. Among the vascular lesions, aneurysm rupture was responsible for bleeding in 6 instances. Arteriovenous malformation was diagnosed in 7 patients. In this subgroup, the maternal and fetal mortality rates were 11.7% and 23.7%, respectively.
Conclusions:We can assert that the association between a brain tumor and vascular lesions with pregnancy is a very unusual event, which jeopardizes both the lives of the mother and infant. It remains incompletely characterized due to the rare nature of these potentially devastating events. Knowing the exact mechanism responsible for the interaction of pregnancy and with these lesions will improve the treatment of these patients.
Keywords: Arteriovenous malformation, brain tumor, cerebral aneurysm, pregnancy
INTRODUCTION
The occurrence of a brain tumor or intracerebral hemorrhage during pregnancy poses risks to both the mother and infant, and presents unique challenges to the neurosurgeon. There are few data on the risks of pregnancy in women with these diseases and no clear guidelines regarding its management during pregnancy, delivery, and puerperium.[
MATERIALS AND METHODS
Data collection
A retrospective study was performed to evaluate 29 pregnant patients with concomitant intracranial lesions treated between January 1986 and January 2015. We will analyze the two groups of patient's separately; 12 consecutive pregnant women diagnosed with brain tumors and 17 patients with vascular lesion underwent treatment in the Neurosurgery Department at the authors’ institutions. The Obstetrics Department of Hospital Federal dos Servidores do Estado (HFSE) is a reference center for high-risk pregnant patients in the city of Rio de Janeiro. All the postoperative care was carried out at the intensive care unit (ICU), a unit with a long history of high-risk pregnancy management. Medical records, surgical descriptions, imagings studies, and the histopathological material were reviewed, thereby creating a database from which information pertinent to the present study was collected. The intraoperative videos and/or photos of 15 patients were analyzed for nuances of the microsurgical technique. The Neurosurgery Department teamed up with Obstetrics, Psychology, and Anesthesiology Departments, as well as with families and patients themselves, for establishing the procedures. The need for informed consent was waived due to the retrospective nature of the study. The first visit in the pediatric, obstetric, and neurosurgery clinics was approximately 15 days after hospital discharge and then after 2 and 6 months, and thereafter, patients were reexamined at 1-year intervals until the fifth postoperative year. The Glasgow Outcome Scale (GOS) defined the neurological outcome. The mengiomas and astrocytomas tumors grading are consistent with the World Health Organization (WHO) classification.
In this series, we performed 9 craniotomies during pregnancy (5 for tumors and 4 for vascular lesions). In all cases, a meticulous neurosurgical dissection with microsurgical removal of the tumor or aneurysms occlusion was successfully carried out without any deleterious effect on the mother or baby. However, a number of special cautionary steps were taken:
Operative Positioning – The pregnant uterus should not compress the inferior vena cava because this leads to a decrease in the venous return, causing arterial hypotension and shock. The dorsal decubitus position, with a trunk rotation to the left, leaned on a roll, prevents aortocaval compression. When the lesion is located on the posterior fossa, the ventral decubitus position is disallowed Anesthesia – Anesthetic consideration involves safety of the mother and fetus. Nitrous oxide is teratogenic in humans and should be avoided. Hyperventilation was used to diminish cerebral blood volume and to facilitate surgical exposure. A PaCO2 between 28 and 30 mmHg is sufficient to maintain adequate surgical conditions, without interfering in fetal oxygenation. Induced arterial hypotension aiming at reducing bleeding must be cautiously used. When the intraoperative fetal monitoring reveals fetal suffering caused by arterial hypotension or hypoxia, it is the precise moment for the systemic arterial pressure to be adjusted. The use of Manitol between 0.5 and 1 g/kg does not usually cause adverse effects to the hydric balance of the fetus Medications – Corticosteroids were cautiously prescribed during pregnancy to control cerebral edema and intracranial hypertension, as well as to accelerate pulmonary fetal maturity. Their prolonged use during pregnancy may result in neonatal hypoadrenalism. Convulsion causes hypoxia and fetal acidosis, and therefore, anticonvulsants’ benefits are probably greater than the possible teratogenic effects and they were used either for treatment or prophylaxis in patients at risk of developing a convulsive crisis. The anticonvulsants should be closely monitored during pregnancy and puerperium.
RESULTS
Neoplastic lesions
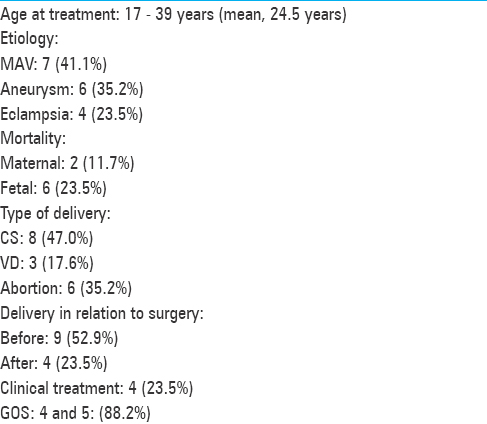
There were 12 patients with ages varying from 25 to 37 years (average, 31 years). The gestational age varied from 16 to 40 weeks (average, 29.7 weeks). The symptoms, location of the tumors, surgical resection extension, and other pertinent information are presented in
Figure 1
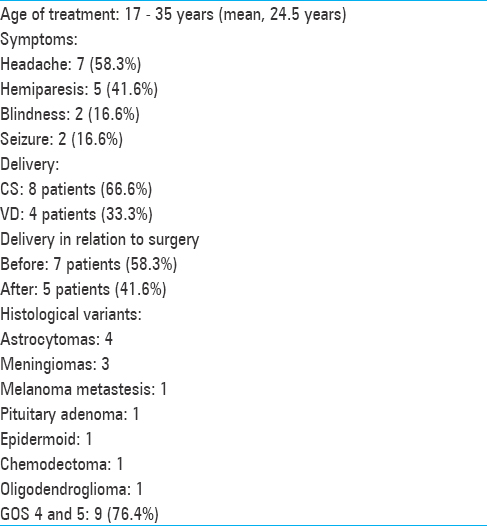
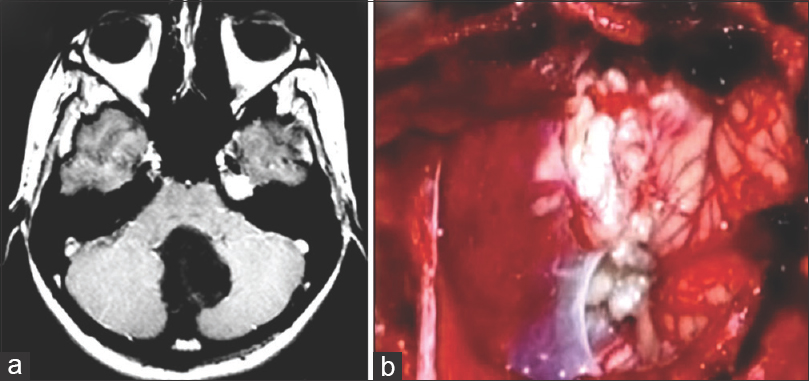
(a) Axial T1-weighted MRI image revealing hypointense lesion located in the cerebellar vermis with invasion of the four ventricule. (b) Operative view showing epidermoid tumor in the magnum cistern. The patient was operated on after CS during the 38th gestational week in a semi-sitting position
Vascular lesions
The patients’ ages varied from 17 to 39 years (average, 28 years). Six patients harbored intracranial aneurysms, 6 harbored AVMs, and 1 patient presented cerebral cavernous malformation (CCM) [Figures
Figure 3
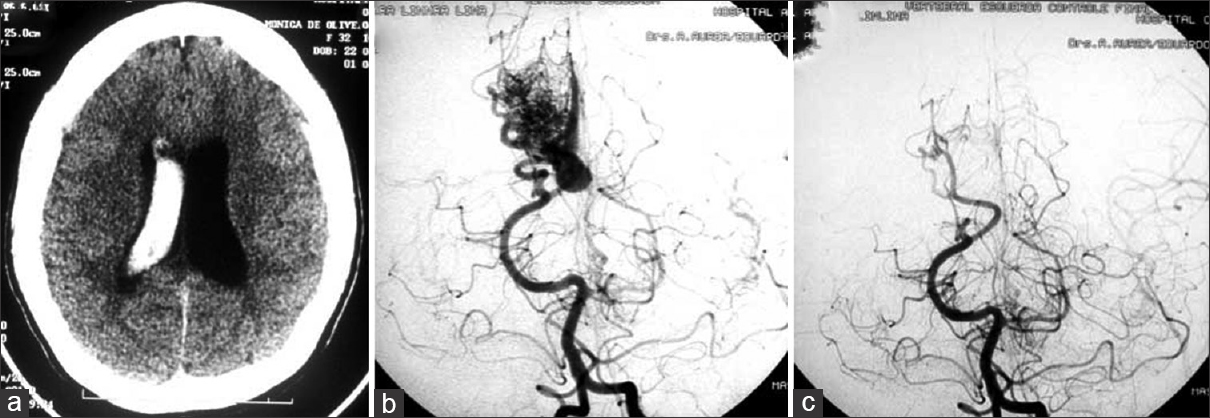
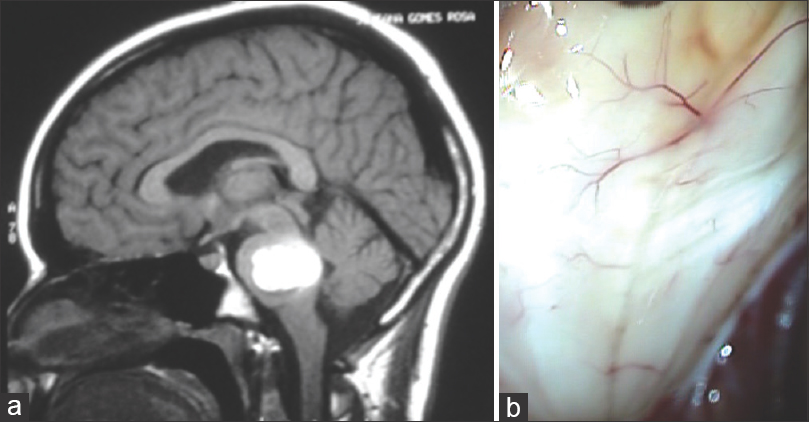
(a) Axial CT scans detecting a right intraventricular hematoma in a second trimester pregnant patient. (b) Cerebral angiography revealed a small AVM fed by the angular artery. (c) Operative image displaying a large draining vein. (d) Postoperative angiography showing the complete exclusion of the lesion. The surgery was performed during pregnancy
Two patients, although undergoing prompt surgical removal of massive intracerebral hematomas, had an unfavorable evolution. Among the 4 patients with eclampsia, two died due to aspiration complication and sepsis. We documented 6 fetal deaths. The presenting symptoms and other clinical features are summarized in
DISCUSSION
Neoplastic and vascular lesions in pregnant patients have some features in common along with some differences; understanding these differences and nuances is essential to choose the most appropriate medical treatment for these patients.
Neoplastic lesions
The occurrence of a brain tumor during pregnancy is a rare event.[
Treatment should always preserve the mother's life and, secondarily, the life of the embryo. Analyzing the aforementioned factors, we could establish five different clinical scenarios:
If the patient presents stable neurological conditions, the neurosurgery may be deferred until gestational period reaches the 30th week. After that, the delivery can be either a VD or a CS, depending exclusively on the obstetric criteria. The craniotomy in this situation is an elective procedure to be performed after the delivery. This was the case of 3 patients If the patient presents with brain edema, but is neurologically stable, cortisone is prescribed to control the cerebral edema as well as to accelerate fetal lung maturity. When the fetus lungs are mature, delivery is performed, followed by an elective craniotomy for tumor removal. This was the procedure for 2 patients If aggravation of neurological symptoms occurs during the fetal maturation period, craniotomy is performed immediately. CS is done just before or after craniotomy. This was an alternative for 1 patient When the patient presents with sudden or severe neurological conditions or has intracranial hypertension, surgery is immediately performed to save the life of the mother. Both gestation and delivery will have routine postoperative follow-up. This was the case of 3 patients If a malignant glioma is suspected, a microsurgical tumor removal should be performed as soon as possible, regardless of gestational age, and not delayed until the postpartum period with risk of disease progression in the meantime.[
Vascular lesions
SAH is rare among pregnant women and the occurrence rate varies from 0.002 to 0.05% of all pregnancies.[
The appropriate treatment in pregnant patients with MAV is conflicting.[
Kalani and Zabramski[
In the future, better-powered studies will hopefully enable more high-level recommendations for managing patients with neoplastic or vascular brain lesions presenting during pregnancy.
CONCLUSIONS
We can assert that the association between a brain tumor and vascular lesions during pregnancy is an unusual event, which jeopardizes both the life of the mother and infant and creates challenges of a neurosurgical, obstetric, and ethical nature. Therefore, a multidisciplinary approach should be used for their care. When aneurysm SAH occurs, microsurgery clipping or endovascular occlusion of the aneurysm is prescribed at any age of the pregnancy. If the bleeding is due to an AVM rupture, the best procedures are yet to be defined, and the therapeutic choice should be made on a case-by-case basis. In a patient with a brain tumor, surgery may be deferred until gestational period reaches at least its 30th week. If the patient presents with neurological deterioration, surgery is immediately performed to save the mothers’ life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albert RJ, Morrison CJ. Neurologic diseases in pregnancy. Obstet Gynecol Clin N Am. 1992. 19: 765-81
2. Amias GA. Cerebral Vascular Disease in pregnancy: I. Hemorrhage. J Obstet Gynaecol Br Comn. 1970. 77: 100-20
3. Barno A, Freeman DW. Maternal deaths due to spontaneous subarachnoid hemorrhage. Am J Obstet Gynecol. 1976. 125: 384-92
4. Blaauw G, Blankenstein MA, Lamberts SW. Sex steroid receptors in human meningiomas. Acta Neurochir. 1986. 79: 42-7
5. Cannell ED, Botterel HE. Subarachoid hemorrhage and pregnancy. Am J Obstet Gynecol. 1956. 72: 844-55
6. Cohen-Gadol AA, Jonathan A, Friedman JA, Friedman JD, Tubbs RS, Munis JR. Neurosurgical management of intracranial lesions in the pregnant patient: A 36-year institutional experience and review of the literature. Clinical article. Neurosurgery. 2009. 111: 1150-7
7. Copelan El, Mabon RF. Spontaneous intracranial bleeding in pregnancy. Obstet Gynaecol. 1967. 20: 373-8
8. Daane TA, Tanty RW. Rupture of congenital intracranial aneurysms in pregnancy. Obstet Gynecol. 1960. 15: 305-14
9. Day J, Batjer HH, Loftus M.editors. Operative positioning and fetal monitoring consideration. Neurosurgical aspect of pregnancy. Illinois: The American Association Neurological Surgeons; 1996. p. 177-8
10. Dias MS, Sekhar LN. Intracranial Hemorrhage from Aneurysms and Arteriovenous Malformation During Pregnancy and the Puerperium. Neurosurgery. 1990. 27: 855-66
11. Donaldson JO. Neurologic emergencies in pregnancy. Obstet Gynaecol Clin N Am. 1991. 18: 199-212
12. Ducray F, Colin P, Cartalat-Carel S. Management of malignant gliomas diagnosed during pregnancy. Rev Neurol. 2006. 162: 322-92
13. Erickson ML, Johnson R, Bannykh SI, Lotbiniere A, Kim JH. Malignant rhabdoide tumor in a pregnant adult female: Literature review of central nervous system rhabdoide tumors. J Neuro-Oncol. 2005. 74: 311-9
14. Eriks AL, Scheithauer BW, Yachnis AT, Fischer BR, Chicoine MR, Werner P. Meningiomas in Pregnancy: A Clinicopathologic Study of 17 Cases. Neurosurgery. 2012. 71: 951-96
15. Grunberg SM, Daniels AM, Muensch H, Daniels JR, Bernstein L, Kortea V. Correlation of meningioma hormone receptor status with hormone sensitivity in a tumor stem-cell assay. J Neurosurg. 1987. 66: 405-8
16. Haba Y, Twyman N, Thomas SJ, Overton C, Dendy P, Burnet NG. Radiotherapy for glioma during pregnancy: Fetal dose estimates, risk assessment and clinical management. Clin Oncol. 2004. 16: 210-4
17. Horton JC, Chambers WA, Lyons SL, Adams RD, KJellberj RN. Pregnancy and the Risk of hemorrhage from cerebral Arteriovenous malformations. Neurosurgery. 1990. 27: 867-72
18. Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: Prognostic considerations. Neurosurgery. 1997. 86: 113-20
19. Isla A, Alvarez F, Gonzalez A, Garcia-Grande A, Perez-Alvarez M, Garcia-Blazquel M. Brain Tumor and Pregnancy. Obstet Gynecol. 1997. 89: 19-22
20. Jayasekera BAP, Bacon AD, Peter C, Whitfield PC. Management of glioblastoma multiforme in pregnancy. A review. J Neurosurg. 2012. 116: 1187-94
21. Kalani MYS, Zabramski JM. Risk for symptomatic hemorrhage of cerebral cavernous malformations during pregnancy. Clinical article. J Neurosurg. 2013. 118: 50-5
22. Kasper EM, Hess PE, Silasi M, Lim K, Gray J, Reddy H, Gilmore L. A pregnant female with a large intracranial mass: Reviewing the evidence to obtain management guidelines for intracranial meningiomas during pregnancy. Surg Neurol Int. 2010. 1: 95-
23. Kim YW, Neal D, Hoh BL. Cerebral Aneurysms in Pregnancy and Delivery: Pregnancy and Delivery Do Not Increase the Risk of Aneurysm Rupture. Neurosurgery. 2013. 72: 143-50
24. Laws ER, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J. Neurosurg. 1984. 61: 665-73
25. Lesch KP, Fahlbusch R. Simultaneous estradiol and progesterone receptor analysis in meningiomas. Surg Neurol. 1986. 26: 257-63
26. Mamelak AN, Withers GJ, Wang X. Choriocarcinoma brain metastasis in a patient with viable intrauterine pregnancy. J Neurosurg. 2002. 97: 477-81
27. Markwalder TM, Zava DT, Goldhirsch A, Markwalder RV. Estrogen and progesterone receptors in meningiomas in relation to clinical and pathologic features. Surg Neurol. 1983. 20: 42-7
28. McCormack BM, Miller DC, Budzilovich GN, Voorhees GJ, Ransohoff J. Treatment and survival of low-grade astrocytoma in adults – 1977–1988. Neurosurgery. 1992. 31: 636-42
29. Michelsen JJ, New PFJ. Brain tumor and pregnancy. J Neurol Neurosurg Psychiatr. 1969. 32: 305-7
30. Molitch ME. Pituitary tumors and pregnancy. Growth Horm IGF Res. 2003. Suppl A: 38-44
31. Nossek E, Ekstein M, Rimon E, Kupferminc MJ, Ram Z. Neurosurgery and pregnancy. Acta Neurochir. 2011. 153: 1727-35
32. Ravindra VM, Braca JA, Jensen RL, Duckworth EA. Management of intracranial pathology during pregnancy: Case example and review of management strategies. Surg Neurol Int. 2015. 6: 43-
33. Robinson JL, Hall CS, Sedzimir CB. Arteriovenous Malformations, Aneurysms and Pregnancy. J Neurosurg. 1974. 41: 63-70
34. Sawin PD, Loftus CM.editors. Spontaneous subarachnoid hemorrhage in pregnancy and the puerperium. Neurosurgical Aspects of Pregnancy. Illinois: American Association of Neurological Surgeons; 1966. p. 85-99
35. Shorshar T, Lamy C, Mas JL. Incidence and causes of strokes with pregnancy and puerperium. Study in public hospitals of the Isle de France. Stroke. 1995. 26: 930-9
36. Simon SL, Moonis G, Judkins AR, Scobie J, Burnett MG, Rhiina HA. Intracranial capillary hemangioma: Case report and review of the lierature. Surg Neurol. 2005. 64: 154-9
37. Tewari KS, Cappuccini F, Asrat T, Flamm BL, Carpenter SE, Disaia PL. Obstetric emergencies precipitated by malignant brain tumors. Am J Obstet Gynecol. 2000. 182: 1215-21
38. Tuncali B, Aksun M, Katircioglu K, Akkol I, Savaci S. Intraoperative fetal heart rate monitoring during emergency neurosurgery in a parturient. J Anesth. 2006. 20: 40-3
39. Verheecke M, Halaska MJ, Lok CA, Ottevanger PB, Petronella B, Fruscio R. Primary brain tumours, meningiomas and brain metastases in pregnancy: Report on 27 cases and review of literature. EJC. 2014. 50: 1462-71
40. Vougioukas V, Kyroussis G, Gläsker S, Tatagiba M, Scheufler KM. Neurosurgical interventions during pregnancy and the puerperium: Clinical considerations and management. Acta Neurochir. 2004. 146: 1287-92