- Department of Physiology, Wayne State University School of Medicine, Detroit, Michigan, USA
Correspondence Address:
Michael D. Wider
Department of Physiology, Wayne State University School of Medicine, Detroit, Michigan, USA
DOI:10.4103/2152-7806.194147
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael D. Wider. Metabolic syndrome and the hepatorenal reflex. 15-Nov-2016;7:99
How to cite this URL: Michael D. Wider. Metabolic syndrome and the hepatorenal reflex. 15-Nov-2016;7:99. Available from: http://surgicalneurologyint.com/surgicalint_articles/metabolic-syndrome-hepatorenal-reflex/
Abstract
Insufficient hepatic O2 in animal and human studies has been shown to elicit a hepatorenal reflex in response to increased hepatic adenosine, resulting in the stimulation of renal as well as muscle sympathetic nerve activity and activating the renin angiotensin system. Low hepatic ATP, hyperuricemia, and hepatic lipid accumulation reported in metabolic syndrome (MetS) patients may reflect insufficient hepatic O2 delivery, potentially accounting for the sympathetic overdrive associated with MetS. This theoretical concept is supported by experimental results in animals fed a high fructose diet to induce MetS. Hepatic fructose metabolism rapidly consumes ATP resulting in increased adenosine production and hyperuricemia as well as elevated renin release and sympathetic activity. This review makes the case for the hepatorenal reflex causing sympathetic overdrive and metabolic syndrome in response to exaggerated splanchnic oxygen consumption from excessive eating. This is strongly reinforced by the fact that MetS is cured in a matter of days in a significant percentage of patients by diet, bariatric surgery, or endoluminal sleeve, all of which would decrease splanchnic oxygen demand by limiting nutrient contact with the mucosa and reducing the nutrient load due to loss of appetite or dietary restriction.
Keywords: Bariatric, cholesterol, diabetes, hepatorenal, metabolic syndrome, obesity, sympathetic
INTRODUCTION
Obesity is increasing rapidly on a global scale and is associated with comorbidities that require expensive medical care and limit the life span,[
While not all obese people develop MetS, the rising incidence of obesity is regarded as an epidemic due to the broad spectrum of associated comorbidities in many patients, including increased mortality, T2DM, glucose intolerance, insulin resistance, hypertension, dyslipidemia, nephropathy with proteinuria, cardiovascular disease, obstructive sleep apnea, nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatotic hepatitis (NASH), polycystic ovary syndrome, and an increased risk of a number of cancers.
The term MetS, or originally Syndrome X, was proposed to foster a coherent clinical approach to management and therapeutic intervention. Though the diagnostic criteria for MetS has been variably defined in the literature, most definitions now include the presence of at least three of the following: Abdominal obesity, insulin resistance, hypertension, elevated fasting plasma glucose, high serum triglycerides, and low high-density lipoprotein levels. A requirement of insulin resistance and abdominal adiposity as part of the diagnostic criteria depends on the group or agency proposing the definition. There have been several attempts to develop a unified set of diagnostic criteria, and in 2009 the International Diabetes Federation, the American Heart Association, and the National Heart, Lung and Blood Institute developed a list of criteria that is now broadly accepted.
METABOLIC SYNDROME ETIOLOGY
A large number of clinical studies have demonstrated that a significant percentage of patients with MetS have durable remission of comorbidities within days of bariatric surgery, calorie restriction (diet), or implantation of an endoluminal plastic sleeve that prevents nutrient contact with the proximal gastrointestinal mucosa, as discussed below. It is essential then to ask of any proposed etiologic factor whether, first, it is capable of causing the spectrum of comorbidities, and second, that it is rapidly eliminated by reducing nutrient contact with the proximal gut.
The theories proposed to explain the dramatic impact of surgical intervention include neuroendocrine, immunologic, and hormonal influences from the proximal gut (foregut theory) and distal gut (hindgut theory).[
It is not the intent of this review to argue the value or relevance of the extensive body of work and related theories for the etiology of MetS but rather to propose an etiologic mechanism based on nutrient contact with the gastrointestinal mucosa in patients with immediate resolution. There are a number of excellent reviews detailing the evidence both for and against the role of gastrointestinal hormones including insulin and GLP-1,[
It is possible, if not probable, that there are multiple pathophysiologic mechanisms involved in the individual morbidities grouped into the classification of MetS. Patients whose comorbidities are resolved in a matter of days, however, may have a unique mechanism related to nutrient contact. The diversity of morbidities and the immediate resolution in up to half of the patients indicates a rapidly acting physiologic mechanism with the potential for broad impact that points to neurologic origin.
SYMPATHETIC OVERDRIVE
Obesity and the related T2DM and MetS have been shown to have a high correlation with elevated sympathetic nerve activity in the kidney (rSNA) and muscles (mSNA)[
Elevated sympathetic discharge following a meal has been reported in normal humans and animals[
Although results of studies on the role of sympathetic nerve activity in relation to vascular response and insulin action are mixed,[
HEPATORENAL REFLEX
The close functional relationship between the liver and kidney provides a potential mechanism for the development of sympathetic overdrive in response to a hepatorenal reflex.[
The reflex nature of the response to low hepatic blood flow is supported by denervation of the liver and/or kidney that has been shown to decrease rSNA and improve renal blood flow and Na+ excretion.[
Regardless of the cause of the elevated mSNA and rSNA observed in HRS and cirrhosis, it has been shown, as stated above, that acute reduction of blood flow or increased hepatic resistance in animals and humans causes rapid stimulation of rSNA resulting in renal vasoconstriction and reduced kidney function with stimulation of the RAS. Intraportal glutamine and serine have also been shown to increase rSNA by causing hepatocyte swelling that reduces sinusoidal blood flow. Cutting the vagal hepatic nerves or spinal transection prevented the effect on rSNA in these experiments and unilateral renal denervation prevented the effect only in the denervated kidney, firmly demonstrating the reflex nature of the response.[
Hepatic adenosine has been identified as a potential factor in stimulating the hepatorenal reflex in that infusion into the portal vein in animals results in an immediate increase in rSNA and a reduction in renal blood flow that is prevented by liver denervation and intraportal, but not intravenous, A1 adenosine receptor blockers.[
HEPATIC OXYGEN DELIVERY
Portal blood flow to the liver increases over 100% following a meal[
While hepatic perfusion is relatively constant over the day, the distribution of blood supply and hence oxygen delivery to the hepatic parenchyma in normal humans and animals results in what is termed “metabolic zonation” involving a periportal Zone 1 (portal inflow) to perivenous Zone 3 (outflow to the hepatic vein). Hepatic oxygen levels vary across the lobule with mixed portal and arterial blood in the Zone 1 periportal region reported to be 60–65 mmHg in animals whereas perivenous Zone 3 O2 is 30–35 mmHg.[
Oxygen delivery to the liver is compromised in obesity by hepatocyte swelling from lipid accumulation. Intracellular lipid follows the same perivenous distribution as the intrahepatic zonal O2 gradient,[
RELATIVE HEPATIC HYPOXIA IN METABOLIC SYNDROME
Low hepatic ATP and inorganic phosphate (Pi) have been reported in MetS and T2DM patients but not in BMI matched, healthy controls and is associated with NAFLD, hepatic insulin resistance, and hyperuricemia.[
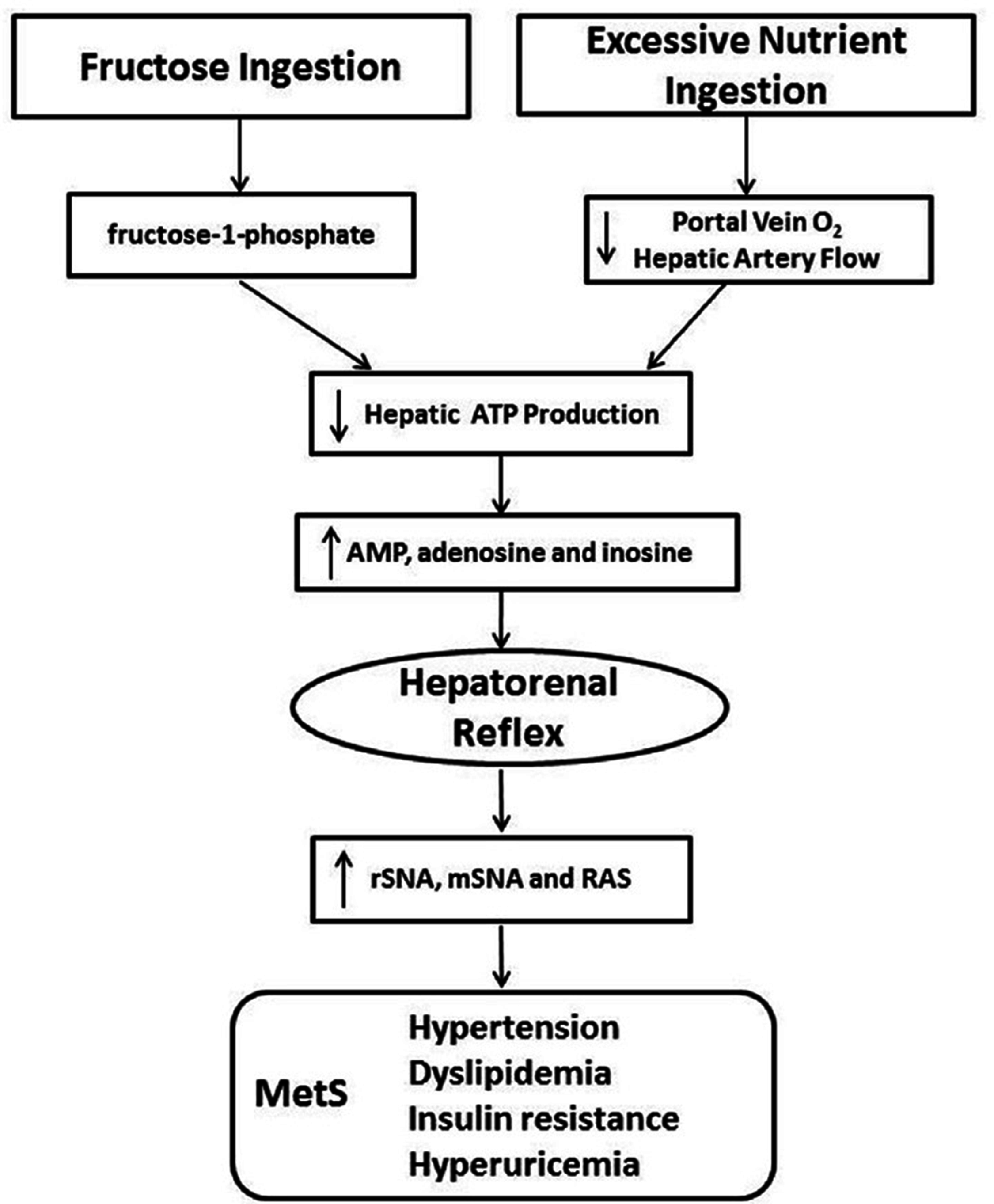
Figure 1
The postulated etiologic mechanisms is supported by the fact that excessive eating and fructose ingestion, both of which can result in MetS, have the potential to reduce hepatic ATP production, increasing levels of adenine nucleotides that are known to stimulate the hepatorenal reflex and theoretically lead to MetS
Reduced hepatic oxygen in rat and mouse hepatocytes has been shown to increase the dephosphorylation of AMP to adenosine, though adenosine is not always an intermediate in adenine nucleotide metabolism. AMP is catabolized by AMP deaminase to inosine monophosphate in the inosine pathway, which would circumvent the production of adenosine.[
This proposed theory of decreased hepatic ATP leading to increased adenosine formation and ultimately MetS is further supported by experimental models where MetS is induced by a high fructose diet.[
Interestingly, BMI has been reported to be inversely correlated with hepatic ATP in normal humans and multiple regression analysis has identified waist circumference as an independent predictor of hepatic ATP flux and Pi concentrations.[
BARIATRIC SURGERY IMPACT ON HEPATIC O2
If a hepatorenal reflex in response to relative hepatic hypoxia is the primary stimulus to sympathetic overdrive and subsequent MetS, then the question of why bariatric surgery, diet, or endoluminal sleeve should correct the hypoxia is central to understanding the role they play in remission. The excessive eating that leads to obesity produces a constant state of increased splanchnic oxygen demand and decreased hepatic artery blood flow that may be significantly corrected by limiting nutrient exposure to the stomach and intestines.
Surgical restructuring of the gut referred to as “bariatric” or “metabolic” surgery includes a number of approaches that were originally focused on weight loss and were designed to either reduce the nutrient load or limit absorption by the small intestine. While these procedures restructure the gut in various ways, all of them result in comorbid disease remission including T2DM[
The one common facet to all the procedures is that they reduce nutrient load and contact with the proximal gastrointestinal mucosa by diversion of nutrient flow and loss of appetite. Further, the surgical placement of a plastic, endoluminal sleeve in the gastroduodenal lumen, preventing proximal mucosal contact with nutrient, has been shown to result in rapid remission, suggesting that mucosal contact is etiologic.[
Bariatric procedures include gastroplasty, biliopancreatic diversion, duodenal switch, biliopancreatic diversion with duodenal switch, Roux-en-y gastric bypass (RYGB), sleeve gastrectomy, vertical gastric banding and adjustable gastric band,[
Both gastrectomy and diversion of the stomach and/or proximal intestine from nutrient contact would significantly lower splanchnic O2 demand resulting in increased portal O2 that may result in increased ATP production, as suggested by the fact that hyperuricemia is reduced following bariatric surgery.[
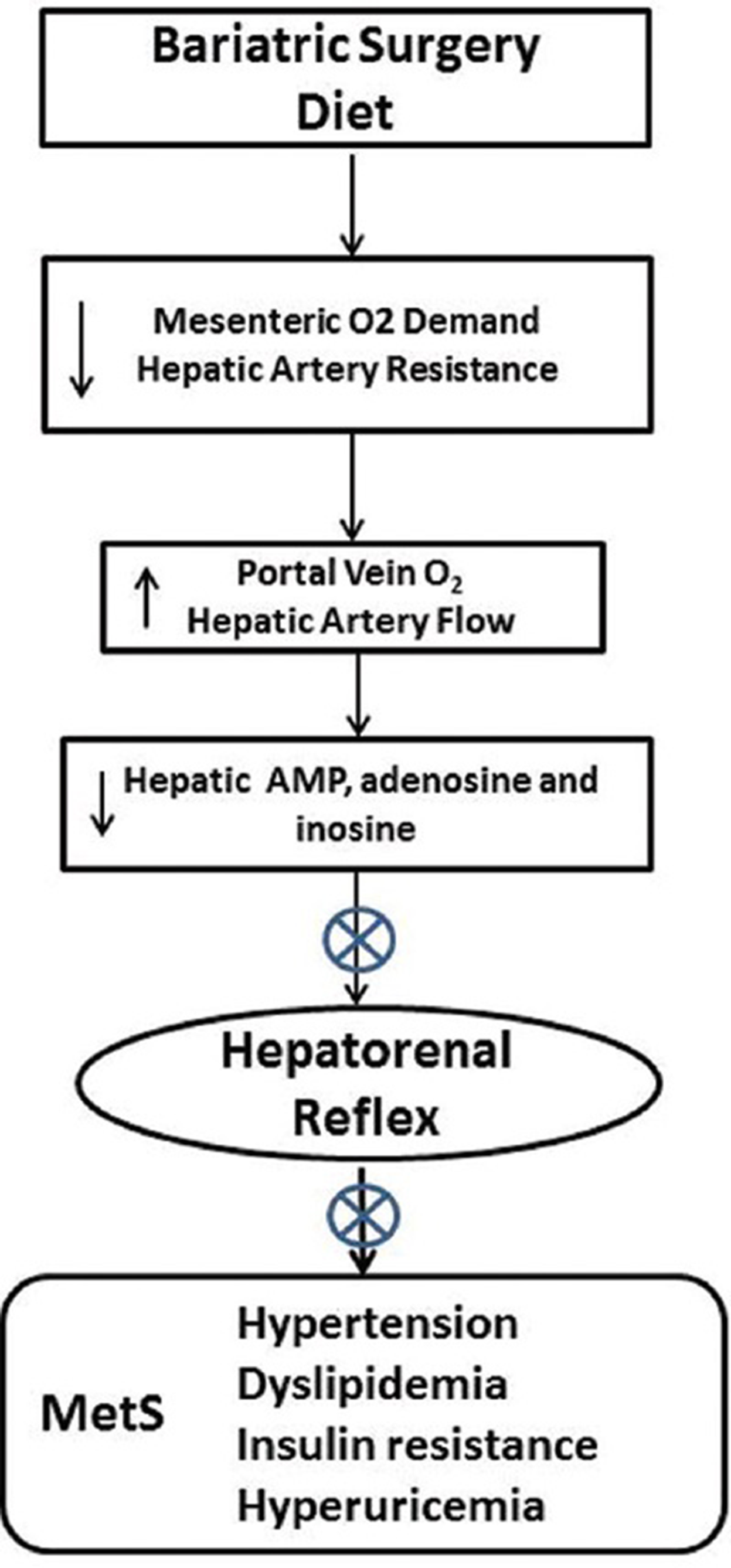
Figure 2
Decreasing the contact of nutrient with the proximal gut by diet or bariatric surgery (including endoluminal sleeve placement) would be expected to reduce enteric oxygen consumption and improve O2 delivery to the liver, potentially enhancing hepatic ATP production and reducing adenine nucleotide accumulation and the hepatorenal reflex
While the stomach and duodenum are not removed in a gastric bypass or RYGB, reduced acid secretion and gastrin release that would lead to O2 consumption by the excluded stomach in humans has been reported.[
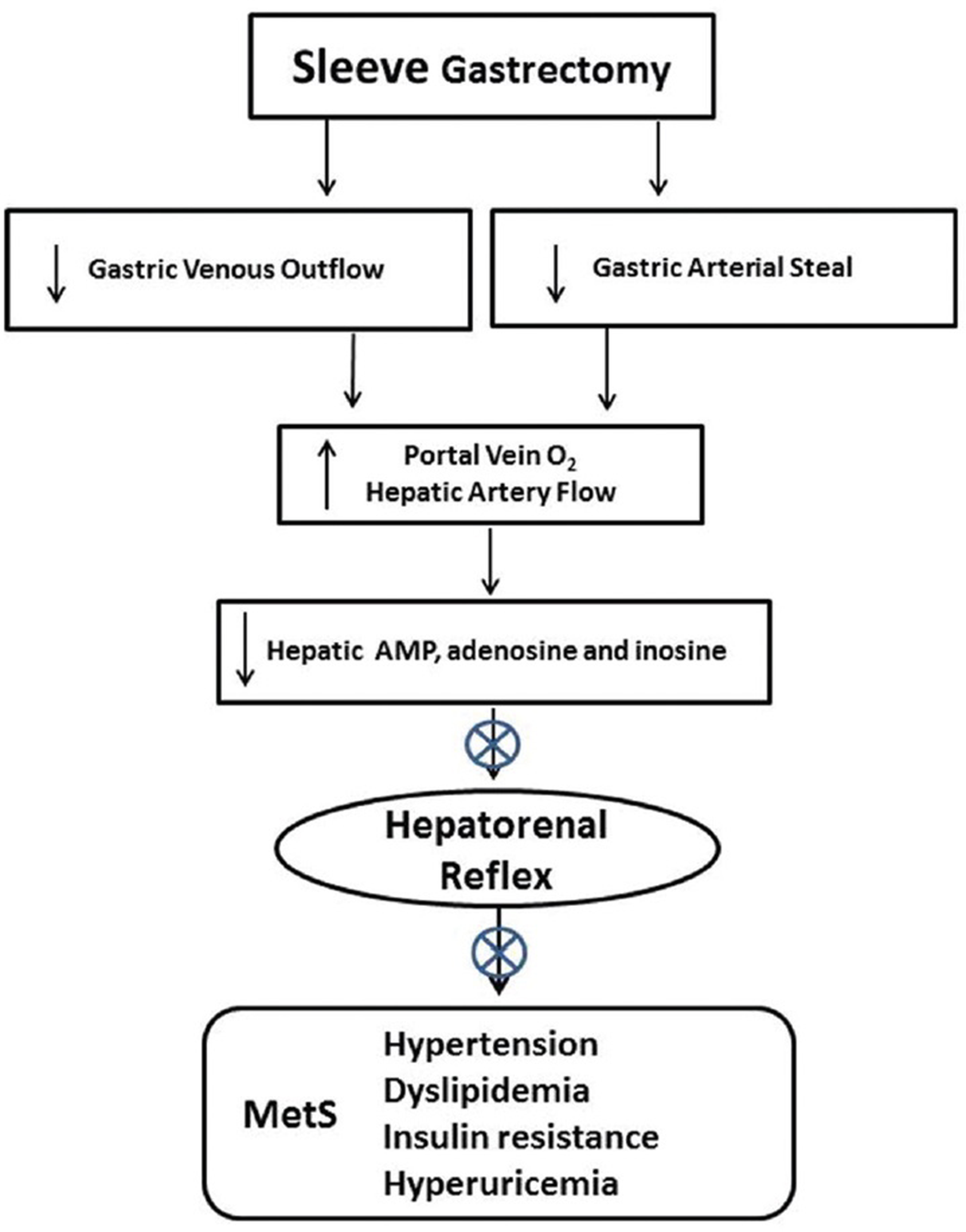
Figure 3
Reduced blood flow in the gastric artery and gastric vein following gastrectomy has the potential to improve O2 delivery to the liver by decreasing low O2 gastric vein contribution and increasing hepatic artery flow by limiting gastric arterial steal from the celiac artery, theoretically allowing increased hepatic ATP production and reducing adenine nucleotide accumulation and the hepatorenal reflex
The immediate resolution of MetS following surgery or endoluminal sleeve would also be significantly impacted by the decreased appetite following bariatric surgery, which is a common problem requiring lifelong counseling and follow-up to insure adequate nutrition and vitamin intake. The reduced eating would further limit splanchnic O2 consumption, improving hepatic O2 delivery and increasing ATP production.
CONCLUSIONS
This review postulates that excessive and/or repetitive eating that produces obesity causes a state of chronic, relative hypoxia in the liver due to lowered O2 in portal blood, reduced hepatic artery flow, and increased hepatic resistance from lipid accumulation and hepatocyte swelling. The resulting low hepatic ATP production leads to the accumulation of adenine nucleotides in the liver that stimulates the hepatorenal reflex producing sympathetic overdrive. Elevated sympathetic outflow has been shown to causes insulin resistance, hypertension, and dyslipidemia and is implicated in other related morbidities such as ventricular hypertrophy, Na+ retention, glucose intolerance, nephropathy with proteinuria, cardiovascular disease, NAFLD, and an increased risk of cancer. Bariatric surgery, diet, and endoluminal sleeve limit the contact of nutrients with the gastrointestinal mucosa as well as decreasing appetite, resulting in increased splanchnic O2 delivery to the liver and preventing the hepatorenal reflex. The fact that some obese patients develop MetS while others do not indicates that MetS is not caused by excess adiposity, but begs the question of what is different between these cohorts, both of which eat excessively and hence should have relative hepatic hypoxia. Vascular anatomy, metabolic response, 2,3-DPG levels, or sensitivity to the hepatorenal reflex are some of the potential areas for further investigation.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Does not apply.
Financial support and sponsorship
Nil.
Conflicts of interest
The author and his institution did not receive any funding or other monetary support for any aspect of the submitted work. The author has received no payment for services and has no financial relationships or intellectual property relevant to the work. The author has no other relationships that would influence or give the appearance of potentially influencing the work.
References
1. Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F. Long term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010. 24: 1005-10
2. Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A. Fatty Liver Subgroup of Look AHEAD Research Group. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012. 56: 952-60
3. Abdulla MH, Sattar MA, Johns EJ. The relation between fructose induced metabolic syndrome and altered renal hemodynamics and excretory function in the rat. Int J Nephrol 2011. 2011. p. 934659-
4. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States 2003-2012. JAMA. 2015. 313: 1973-4
5. Aguirre V, Stylopoulos N, Grinbaum R, Kaplan LM. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity. 2008. 16: 2585-92
6. Alexandrides TK, Skroubis G, Kalfarentzos F. Resolution of diabetes mellitus and metabolic syndrome following Roux-en-Y gastric bypass and a variant of biliopancreatic diversion in patients with morbid obesity. Obes Surg. 2007. 17: 176-84
7. Ali MR, Fuller WD, Rasmussen J. Detailed description of early response of metabolic syndrome after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009. 5: 346-51
8. Alkharouf J, Nalinikumari K, Corry D, Tuck M. Long-term effects of the angiotensin converting enzyme inhibitor captopril on metabolic control in non-insulin-dependent diabetes mellitus. Am J Hypertens. 1993. 6: 337-43
9. Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008. 28: 81-95
10. Arroyo V, Fernandez J. Management of hepatorenal syndrome in patients with cirrhosis. J Nat Rev Nephrol. 2011. 7: 517-26
11. Assaife-Lopes N, Wengert M, de Sá Pinheiro AA, Leão-Ferreira LR, Caruso-Neves C. Inhibition of renal Na+-ATPase activity by inosine is mediated by A1 receptor-induced inhibition of the cAMP signaling pathway. Arch Biochem Biophys. 2009. 489: 76-81
12. Astiarraga B, Gastaldelli A, Muscelli E, Baldi S, Camastra S, Mari A. Biliopancreatic diversion in nonobese patients with type 2 diabetes: Impact and mechanisms. J Clin Endocrinol Metab. 2013. 98: 2765-73
13. Axelsen LN, Lademann JB, Petersen JS, Holstein-Rathlou NH, Ploug T, Prats C. Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocardial lipid accumulation. Am J Physiol Regul Integr Comp Physiol. 2010. 298: R1560-70
14. Baba T, Kodama T, Ishizaki T. Effect of chronic treatment with enalapril on glucose tolerance and serum insulin in non-insulin-resistant Japanese patients with essential hypertension. Eur J Clin Pharmacol. 1993. 45: 23-7
15. Balci A, Karazincir S, Sumbas H, Oter Y, Egilmez E, Inandi T. Effects of diffuse fatty infiltration of the liver on portal vein flow hemodynamics. J Clin Ultrasound. 2008. 36: 134-40
16. Ballantyne GH, Farkas D, Laker S, Wasielewski A. Short term changes in insulin resistance following weight loss surgery for morbid obesity: Laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006. 16: 1189-97
17. Barbash IM, Waksman R. Sympathetic renal denervation; hypertension beyond SYMPLICITY. Cardiovasc Revascular Med. 2013. 14: 229-35
18. Barsotti C, Ipata PL. Metabolic regulation of ATP breakdown and of adenosine production in rat brain extracts. Int J Biochem Cell Biol. 2004. 36: 2214-25
19. Batchelder AJ, Williams R, Sutton C, Khanna A. The evolution of minimally invasive bariatric surgery. J Surg Res. 2013. 183: 559-66
20. Bayham BE, Greenway FL, Bellanger DE, O’Neil CE. Early resolution of type 2 diabetes seen after Roux-en-Y and vertical sleeve gastrectomy. Diabetes Technol Ther. 2012. 14: 30-4
21. Belloni FL, Elkin PL, Giannotto B. The mechanism of adenosine release from hypoxic rat liver cells. Br J Pharmacol. 1985. 85: 441-6
22. Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration. J Clin Invest. 1989. 84: 1403-9
23. Bitkin EC, Boyraz M, Taºkın N, Akçay A, Ulucan K, Akyol MB. Effects of ACE inhibitors on insulin resistance and lipid profile in children with metabolic syndrome. J Clin Res Pediatr Endocrinol. 2013. 5: 164-9
24. Blanchart A, Rodriguez-Puyol D, Santos JC, Hernando L, Lopez-Novoa JM. Effect of chronic and progressive hepatic outflow blockade on renal function in rats. J Lab Clin Med. 1987. 109: 718-3
25. Boonchaya-anant P, Apovian CM. Metabolically healthy obesity-does it exist?. Curr Atheroscler Rep. 2014. 16: 441-6
26. Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults. Am J Public Health. 2014. 104: 512-9
27. Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR. Fructose-fed rhesus monkeys: A nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011. 4: 243-52
28. Browse DJ, Mathie RT, Benjamin IS, Alexander B. The role of ATP and adenosine in the control of hepatic blood flow in the rabbit in vivo. Comp Hepatol. 2003. 2: 9-
29. Brundin T, Wahren J. Influence of protein ingestion on human splanchnic and whole-body oxygen consumption, blood flow and blood temperature. Metabolism. 1994. 43: 626-32
30. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K. Bariatric Surgery; a systematic review and meta-analysis. JAMA. 2004. 292: 1724-37
31. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009. 122: 248-56
32. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013. 23: 427-36
33. Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension. 2002. 39: 496-501
34. Carnethon MR, Jacobs DR, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: The CARDIA study. Diabetes Care. 2003. 26: 3035-41
35. Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose induced hypertension. Am J Hypertens. 2003. 6: 973-8
36. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014. 149: 275-87
37. Cheung LY, Moody FG, Larson K, Lowry SF. Oxygen consumption during cimetidine and prostaglandin E2 inhibition of acid secretion. Am J Physiol. 1978. 234: E445-50
38. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007. 120: 442-7
39. Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP. Surgery decreases long-term mortality, morbidity and health care use in morbidly obese patients. Ann Surg. 2004. 240: 416-24
40. Ciarla S, Struglia M, Giorgini P, Striuli R, Necozione S, Properzi G. Serum uric acid levels and metabolic syndrome. Arch Physiol Biochem. 2014. 120: 119-22
41. Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y. Uric acid, the metabolic syndrome and renal disease. J Am Soc Nephrol. 2006. 17: S165-8
42. Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004. 70: 1-4
43. Cohen R, le Roux CW, Papamargaritis D, Salles JE, Petry T, Correa JL. Role of proximal gut exclusion from food on glucose homeostasis in patients with Type 2 diabetes. Diabet Med. 2013. 30: 1482-6
44. Contini S, Pezzarossa A, Corradi A, Scarpignato C. Renal removal of glucagon and insulin after acute hepatic ischemia in dogs. Diabetes Metab. 1983. 9: 3-8
45. Corcelles R, Daigle CR, Schauer PR. Metabolic effects of bariatric surgery. Eur J Emdocrin. 2016. 174: R19-28
46. Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: A pilot study. JAMA. 1999. 282: 1659-64
47. Cox HS, Kaye DM, Thompson JM, Turner AG, Jennings GL, Itsiopoulos C. Regional sympathetic nervous activation after a large meal in humans. Clin Sci. 1995. 89: 145-54
48. Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Rel Dis. 2008. 4: S47-55
49. Dai S, McNeill JH. Fructose induced hypertension in rats is concentration and duration dependent. J Pharmacol Toxicol Methods. 1995. 33: 101-7
50. Daly PA, Landsberg L. Hypertension in obesity and NIDDM. Role of insulin and sympathetic nervous system. Diabetes Care. 1991. 14: 240-8
51. Dauzat M, Lafortune M, Patriquin H, Pomier-Layrargues G. Meal induced changes in hepatic and splanchnic circulation: A noninvasive Doppler study in normal humans. Eur J Appl Physiol. 1994. 68: 373-80
52. Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL. Effectiveness of renal denervation therapy for resistant hypertension: A systematic review and meta-analysis. J Am Coll Cardiol. 2013. 62: 231-41
53. Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009. 33: 116-24
54. De Angelis K, Senador DD, Mostarda C, Irigoyen MC, Morris M. Sympathetic overactivity precedes metabolic dysfunction in a fructose model of glucose intolerance in mice. Am J Physiol Regul Integr Comp Physiol. 2012. 302: R950-7
55. de Jager RL, Blankestijn PJ. Pathophysiology I: The kidney and the sympathetic nervous system. Eurointervention. 2013. 9: R42-7
56. de Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA. Endoscopic duodenal jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013. 23: 1354-60
57. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist to hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur Heart J. 2007. 28: 850-6
58. de Moura EG, Martins BC, Lopes GS, Orso IR, de Oliveira SL, Galvão Neto MP. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diab Tech Ther. 2012. 14: 183-9
59. DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension. 2000. 36: 1083-8
60. Dong M, Ren J. What fans the fire: Insights into mechanisms of leptin in metabolic syndrome associated heart diseases. Curr Pharm Des. 2014. 20: 652-8
61. Edfeldt H, Lundvall J. Sympathetic baroreflex control of vascular resistance in comfortably warm man. Analyses of neurogenic constrictor responses in the resting forearm and in its separate skeletal muscle and skin tissue compartments. Acta Physiol Scand. 1993. 147: 437-47
62. Egan BM. Insulin resistance and the sympathetic nervous system. Curr Hypertens Rep. 2003. 5: 247-54
63. Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J Gastroenterol. 2010. 16: 6046-57
64. Erdogmus B, Tamer A, Buyukkaya R, Yazici B, Buyukkaya A, Korkut E. Portal vein hemodynamics in patients with non-alcoholic fatty liver disease. Tohoku J Exp Med. 2008. 215: 89-93
65. Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D. Weight loss and metabolic improvement in morbidly obese subjects implanted for 12 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg. 2012. 255: 1080-5
66. Escalona A, Yáñez R, Pimentel F, Galvao M, Ramos AC, Turiel D. Initial human experience with restrictive duodenal-jejunal bypass liner for treatment of morbid obesity. Surg Obes Relat Dis. 2010. 6: 126-31
67. Eslami P, Tuck M. The role of the sympathetic nervous system in linking obesity with hypertension in white vs black Americans. Curr Hypertens Rep. 2003. 5: 269-72
68. Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: From obesity to diabetes. Am J Hypertens. 2001. 14: 304S-9
69. Ezzat W, Lautt WW. Hepatic arterial pressure flow autoregulation is adenosine mediated. Am J Physiol. 1987. 252: H836-45
70. Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin Sci. 1994. 86: 159-67
71. Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006. 130: 41-50
72. Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Record. 2008. 291: 684-
73. Floras JS, Legault L, Morali GA, Hara K, Blendis LM. Increased sympathetic outflow in cirrhosis and ascites: Direct evidence from intraneural recordings. Ann Intern Med. 1991. 114: 373-80
74. Foo J, Krebs J, Hayes MT, Bell D, Macartney-Coxson D, Croft T. Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg. 2011. 21: 1914-20
75. Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007. 115: 2526-32
76. Fox CS, Massaro JM, Hoffmann U, Pou KM, Mauroviah-Hovrat P, Liu CY. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007. 116: 39-48
77. Gallavan RH Jr, Chou CC, Kvietys PR, Sit SP. Regional blood flow during digestion in the conscious dog. Am J Physiol. 1980. 238: H220-5
78. Garrido-Sanchez L, Murri M, Rivas-Becerra J, Ocaña-Wilhelmi L, Cohen RV, Garcia-Fuentes E. Bypass of the duodenum improves insulin resistance much more rapidly than sleeve gastrectomy. Surg Obes Rel Dis. 2012. 8: 145-50
79. Garzia P, Ferri GM, Ilardi M, Messina FR, Amoroso A. Pathophysiology, clinical features and management of hepatorenal syndrome. Eur Rev Med Pharmacol Sci. 1998. 2: 181-4
80. Gebhardt R, Matz-Soja M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol. 2014. 20: 8491-504
81. Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008. 52: 605-15
82. Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes: A systematic review. Surg Obes Relat Dis. 2010. 6: 707-13
83. Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomized controlled trials. BMJ. 2013. 347: f5934-
84. Goyal RK. Hyperinsulinemia and insulin resistance in hypertension: Differential effects of antihypertensive agents. Clin Exp Hypertens. 1999. 21: 167-79
85. Granger DN, Holm L, Kvietys P. The gastrointestinal circulation: Physiology and pathophysiology. Comp Physiol. 2015. 5: 1541-83
86. Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell’oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: Evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012. 14: 333-8
87. Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004. 22: 2363-9
88. Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005. 48: 1359-65
89. Grassi G, Quarti-Trevano F, Seravalle G, Dell’Oro R, Dubini A, Mancia G. Differential sympathetic activation in muscle and skin neural districts in the metabolic syndrome. Metabolism. 2009. 58: 1446-51
90. Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M. Sympathetic activation in obese normotensive subjects. Hypertension. 1995. 25: 560-3
91. Grassi G, Seravalle G, Quarti-Trevano F, Mineo C, Lonati L, Facchetti R. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens. 2010. 28: 1313-20
92. Grassi G. Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens Res. 2006. 29: 839-47
93. Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971. 51: 23-65
94. Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut. 2014. 63: 1238-46
95. Haddock RE, Hill CE. Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J Physiol. 2011. 589: 3289-307
96. He F, Rodriguez-Colon S, Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Berg A, Imamura Kawasawa Y. Abdominal obesity and metabolic syndrome burden in adolescents - Penn State Children Cohort Study. J Clin Densitom. 2015. 18: 30-6
97. Hedberg J, Sundstrom J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: Systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obesity Rev. 2014. 15: 555-63
98. Henriksen JH, Ring-Larsen H, Christensen NJ. Aspects of sympathetic nervous system regulation in patients with cirrhosis: A 10-year experience. Clin Physiol. 1991. 11: 293-306
99. Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998. 29: 328-41
100. Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: Mechanism and metabolic consequences. Biochimie. 2014. 96: 121-9
101. Hocher B, Heiden S, von Websky K, Arafat AM, Rahnenführer J, Alter M. Renal effects of the novel selective adenosine A1 receptor blocker SLV329 in experimental liver cirrhosis in rats. PLoS One. 2011. 6: e17891-
102. Høost U, Kelbaek H, Rasmusen H, Court-Payen M, Christensen NJ, Pedersen-Bjergaard U. Haemodynamic effects of eating: The role of meal composition. Clin Sci. 1996. 90: 269-76
103. Hori M, Neto AC, Austen WG, McDermott WV Jr. Isolated in vivo hepatorenal perfusion in the dog. Circulatory and functional response of the kidney to hepatic anoxia. J Surg Res. 1967. 7: 413-7
104. Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003. 108: 3097-101
105. Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose induced insulin resistance and hypertension in rats. Hypertension. 1987. 10: 512-6
106. Hwang LC, Bai CH, Sun CA, Chen CJ. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac J Clin Nutr. 2012. 21: 227-33
107. Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003. 10: 447-56
108. Inabnet WB, Winegar DA, Sherif B, Sarr MG. Early outcomes of bariatric surgery in patients with metabolic syndrome: An analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2012. 214: 550-7
109. Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci. 2012. 109: 4320-5
110. Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012. 22: 1084-96
111. Jakab F, Ráth Z, Schmal F, Nagy P, Faller J. The interaction between hepatic arterial and portal venous blood flows: Simultaneous measurement by transit time ultrasonic volume flowmetry. Hepatogastroenterol. 1995. 42: 18-21
112. Jalan R, Forrest EH, Redhead DN, Dillon JF, Hayes PC. Reduction in renal blood flow following acute increase in the portal pressure: Evidence for the existence of a hepatorenal reflex in man?. Gut. 1997. 40: 664-70
113. Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993. 21: 618-23
114. Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012. 256: 1023-9
115. Jungermann K, Kietzmann T. Oxygen: Modulator of metabolic zonation and disease of the liver. Hepatology. 2000. 31: 255-60
116. Kaaja RJ, Poyohonin-Alho MK. Insulin resistance and sympathetic overactivity. J Hypertens. 2006. 24: 131-41
117. Kalaitzidis RG, Karasavvidou D, Siamopoulos KC. Renal sympathetic denervation and renal physiology. Curr Clin Pharmacol. 2013. 8: 189-96
118. Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes. Diabetes Care. 2013. 36: 2175-82
119. Katz NR, Fischer W, Giffhorn S. Distribution of fatty acid and ketone body metabolism in periportal and perivenous rat-liver tissue. Eur J Biochem. 1983. 135: 103-7
120. Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity and mortality: Impact of cardiorespiratory fitness. Diabetes Care. 2005. 28: 391-7
121. Kaul A, Sharma J. Impact of bariatric surgery on comorbidities. Surg Clin North Am. 2011. 91: 1295-312
122. Khoury N, McGill JB. Reduction in insulin sensitivity following administration of the clinically used low-dose pressor, norepinephrine. Diabetes Metab Res Rev. 2011. 27: 604-8
123. Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol. 2013. 33: 75-89
124. Kohli R, Stefater MA, Inge TH. Molecular insights from bariatric surgery. Rev Endocr Metab Disord. 2011. 12: 211-7
125. Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cellular Endocrinol. 2013. 379: 35-42
126. Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence functional nature of renal failure. N Engl J Med. 1969. 280: 1367-71
127. Kostreva DR, Castaner A, Kampine JP. Reflex effects of hepatic baroreceptors on renal and cardiac sympathetic nerve activity. Am J Physiol. 1980. 238: R390-4
128. Kowalewski K, Kolodej A. Relation between hydrogen ion secretion and oxygen consumption by ex vivo isolated canine stomach, perfused with homologous blood. Can J Physiol Pharmacol. 1972. 50: 955-61
129. Koyama S, Kanai K, Aibiki M, Fujita T. Reflex increases in renal nerve activity during acutely altered portal venous pressure. J Auton Nerv Syst. 1988. 23: 55-62
130. Koyama S, Nishida K, Terada N, Shiojima Y, Takeuchi T. Reflex renal vasoconstriction on portal vein distension. Japan J Physiol. 1986. 36: 441-50
131. Kwon Y, Abdemur A, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg Obes Relat Dis. 2014. 10: 235-42
132. Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y in the Indian population: A retrospective 1 year study. Obes Surg. 2010. 20: 1-6
133. Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007. 50: 862-68
134. Lambert EA, Straznicky NE, Lambert GW. A sympathetic view of human obesity. Clin Auton Res. 2013. 23: 9-14
135. Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome-causes, consequences and therapeutic implications. Pharmacol Ther. 2010. 126: 159-72
136. Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. Am J Hypertens. 1989. 2: 125S-32S
137. Lang F, Tschernko E, Schulze E, Ottl I, Ritter M, Völkl H. Hepatorenal reflex regulating kidney function. Hepatology. 1991. 14: 590-4
138. Lautt WW, Legare DJ. The use of 8-phenyltheophylline as a competitive antagonist of adenosine and an inhibitor of the intrinsic regulatory mechanism of the hepatic artery. Can J Physiol Pharmacol. 1985. 63: 717-22
139. Lautt WW. Control of hepatic and intestinal blood flow: Effect of isovolaemic haemodilution on blood flow and oxygen uptake in the intact liver and intestines. J Physiol. 1977. 265: 313-26
140. Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: Hepatic arterial buffer response. Am J Physiol. 1985. 249: G549-56
141. Lautt WW. Relationship between hepatic blood flow and overall metabolism: The hepatic arterial buffer. Fed Proc. 1983. 42: 1662-6
142. Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol. 1994. 266: E242-7
143. Licht CM, de Geus EJ, Penninx BW. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J Clin Endocrinol Metab. 2013. 98: 2484-93
144. Liew PL, Lee WJ, Lee YC, Wang HH, Wang W, Lin YC. Hepatic histopathology of morbid obesity: Concurrence of other forms of chronic liver disease. Obes Surg. 2006. 16: 1584-93
145. Ligtenberg G, Blankestijn PJ, Oey PL, Klein IH, Dijkhorst-Oei LT. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999. 340: 1321-8
146. Lutz J, Henrich H, Bauereisen E. Oxygen supply and uptake in the liver and the intestine. Pflugers Arch. 1975. 360: 7-15
147. Madsen JL, Søndergaard SB, Møller S. Meal induced changes in splanchnic blood flow and oxygen uptake in middle aged healthy humans. Scand J Gastroenterol. 2006. 41: 87-92
148. Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: A pilot study. Circulation. 2011. 123: 1940-6
149. Majumbder S, Birk J. A review of the current status of endoluminal therapy as a primary approach to obesity management. Surg Endosc. 2013. 27: 2305-11
150. Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007. 25: 909-20
151. Mason EE, Munns JR, Kealey GP, Wangler R, Clarke WR, Cheng HF. Effect of gastric bypass on gastric secretion. Surg Obes Relat Dis. 2005. 1: 155-60
152. Mayer MA, Höcht C, Gironacci M, Opezzo JA, Taira CA, Fernández BE. Hypothalamic angiotensinergic-noradrenergic systems interaction in fructose induced hypertension. Regul Pept. 2008. 146: 38-45
153. McCarty MF. Elevated sympathetic activity may promote insulin resistance syndrome by activating alpha-1 adrenergic receptors on adipocytes. Med Hypotheses. 2004. 62: 830-8
154. Meijer RI, van Wagensveld BA, Siegert CE, Eringa EC, Serné EH, Smulders YM. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: A systematic review. Arch Surg. 2011. 146: 744-50
155. Michalakis K, le Roux C. Gut hormones and leptin: Impact on energy control and changes after bariatric surgery--what the future holds. Obes Surg. 2012. 22: 1648-57
156. Ming Z, Fan YJ, Yang X, Lautt WW. Blockade of intrahepatic adenosine receptors improves urine excretion in cirrhotic rats induced by thioacetamide. J Hepatol. 2005. 42: 680-6
157. Ming Z, Fan YJ, Yang X, Lautt WW. Contribution of hepatic adenosine A1 receptors to renal dysfunction associated with acute liver injury in rats. Hepatology. 2006. 44: 813-22
158. Ming Z, Lautt WW. Intrahepatic adenosine-mediated activation of hepatorenal reflex is via A1 receptors in rats. Can J Physiol Pharmacol. 2006. 84: 1177-84
159. Ming Z, Smyth DD, Lautt WW. Decreases in portal flow trigger a hepatorenal reflex to inhibit renal sodium and water excretion in rats: Role of adenosine. Hepatology. 2002. 35: 167-75
160. Ming Z, Smyth DD, Lautt WW. Intrahepatic adenosine triggers a hepatorenal reflex to regulate renal sodium and water excretion. Auton Neurosci. 2001. 93: 1-7
161. Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009. 35: 518-23
162. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012. 366: 1577-85
163. Moan A, Risanger T, Eide I, Kjeldsen SE. The effect of angiotensin II receptor blockade on insulin sensitivity and sympathetic nervous activity in primary hypertension. Blood Press. 1994. 3: 185-8
164. Moneta GL, Taylor DC, Helton WS, Mulholland MW, Strandness DE. Duplex ultrasound measurement of postprandial intestinal blood flow: Effect of meal composition. Gastroenterology. 1988. 95: 1294-301
165. Moody FG. Oxygen consumption during thiocynate inhibition of gastric acid secretion in dogs. Am J Physiol. 1968. 215: 127-31
166. Moustarah F, Gilbert A, Després JP, Tchernof A. Impact of gastrointestinal surgery on cardiometabolic risk. Curr Atheroscler Rep. 2012. 14: 588-96
167. Muller AF, Fullwood L, Hawkins M, Cowley AJ. The integrated response of the cardiovascular system to food. Digestion. 1992. 52: 184-93
168. Musella M, Susa A, Greco F, De Luca M, Manno E, Di Stefano C. The laparoscopic mini-gastric bypass: The Italian experience: Outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014. 28: 156-63
169. Nair S, P Chacko V, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: Comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003. 98: 466-70
170. Nascimento FP, Macedo-Júnior SJ, Pamplona FA, Luiz-Cerutti M, Córdova MM, Constantino L. Adenosine A1 receptor dependent antinociception induced by inosine in mice: Pharmacological, genetic and biochemical aspects. Mol Neurobiol. 2015. 51: 1368-78
171. Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012. 147: 847-54
172. Neovius M, Linne Y, Rossner S. BMI, waist circumference and waist-to-hip ratio as diagnostic test for fatness in adolescents. Int J Obes. 2005. 29: 163-9
173. Nora M, Guimarães M, Almeida R, Martins P, Gonçalves G, Freire MJ. Metabolic laparoscopic gastric bypass for obese patients with type 2 diabetes. Obes Surg. 2011. 21: 1643-9
174. Noria SF, Grantcharov T. Biologic effects of bariatric surgery on obesity-related comorbidities. Can J Surg. 2013. 56: 47-57
175. Norryd C, Denker H, Lunderquist A, Olin T, Tylén U. Superior mesenteric blood flow during digestion in man. Acta Chir Scand. 1975. 141: 197-202
176. Oberbach A, Neuhaus J, Inge T, Kirsch K, Schlichting N, Blüher S. Bariatric surgery in severely obese adolescents improves major comorbidities including hyperuricemia. Metabolism. 2014. 63: 242-9
177. O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long term outcomes after bariatric surgery: Fifteen year follow up of adjustable gastric banding and a systematic review of the bariatric surgery literature. Ann Surg. 2013. 257: 87-94
178. Palomar R, Fernández-Fresnedo G, Domínguez-Diez A, López-Deogracias M, Olmedo F, Martín de Francisco AL. Effects of weight loss after BPD on metabolism and cardiovascular profile. Obes Surg. 2005. 15: 794-8
179. Pan H, Guo J, Xu Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol Behav. 2014. 130: 157-69
180. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003. 163: 427-36
181. Penesova A, Radikova Z, Cizmarova E, Kvetnansky R, Blazicek P, Vicek M. The role of norepinephrine and insulin resistance in an early stage of hypertension. Ann N Y Acad Sci. 2008. 1148: 490-4
182. Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes and hypertension. Clin Exp Hypertens. 2001. 23: 45-55
183. Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg. 2008. 247: 21-7
184. Perry MA, Bulkley GB, Kvietys PR, Granger DN. Regulation of oxygen uptake in resting and pentagastrin stimulated stomach. Am J Physiol. 1982. 242: G565-9
185. Petrie JL, Patman GL, Sinha I, Alexander TD, Reeves HL, Agius L. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. Am J Physiol Endocrinol Metab. 2013. 305: E1255-65
186. Phillips CM, Dillon C, Harrington JM, McCarthy VJ, Kearney PM, Fitzgerald AP. Defining metabolically healthy obesity: Role of dietary and lifestyle factors. PLoS One. 2013. 8: e76188-
187. Plourde CÉ, Grenier-Larouche T, Caron-Dorval D, Biron S, Marceau S, Lebel S. Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity. 2014. 22: 1838-46
188. Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: Role of the foregut. World J Surg. 2001. 25: 527-31
189. Pories WJ, MacDonald KG, Morgan EJ, Sinha MK, Dohm GL, Swanson MS. Surgical treatment of obesity and its effect on diabetes: 10 year follow-up. Am J Clin Nutr. 1992. 55: 582S-5S
190. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995. 222: 339-52
191. Pories WJ. Why does the gastric bypass control type 2 diabetes mellitus?. Obes Surg. 1992. 2: 303-13
192. Porte D, Robertson RP. Control of insulin by catecholamines, stress and the sympathetic nervous system. Fed Proc. 1973. 32: 1792-6
193. Pournaras DJ, Aasheim ET, Søvik TT, Andrews R, Mahon D, Welbourn R. Effect of the definition of type II diabetes remission on the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012. 99: 100-3
194. Pournaras DJ, leRoux CW. Obesity, gut hormones and bariatric surgery. World J Surg. 2009. 33: 1983-8
195. Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P. Remission of type 2 diabetes after gastric bypass and banding: Mechanisms and 2 year outcomes. Ann Surg. 2010. 252: 966-71
196. Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes. 2011. 35: 971-81
197. Qamar MI, Read AE. Effects of ingestion of carbohydrate, fat, protein and water on the mesenteric blood flow in man. Scand J Gastroenterol. 1988. 23: 26-30
198. Qiao Q, Nyamdorj R. Is the association of T2D with waist circumference or waist to hip ratio stronger than that with body mass index?. Eur J Clin Nutr. 2010. 64: 30-4
199. Raj PP, Kumaravel R, Chandramaliteeswaran C, Vaithiswaran V, Palanivelu C. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: Preliminary results of a prospective series from India. Surg Endosc. 2012. 26: 688-92
200. Rappaport AM. Hepatic blood flow: Morphologic aspects and physiologic regulation. Int Rev Physiol. 1980. 21: 1-63
201. Rappaport AM. The microcirculatory hepatic unit. Microvasc Res. 1973. 6: 212-28
202. Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011. 96: 2525-31
203. Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes Rev. 2014. 15: 781-90
204. Ribeiro MM, Trombetta IC, Batalha LT, Rondon MU, Forjaz CL, Barretto AC. Muscle sympathetic nerve activity and hemodynamic alterations in middle aged women. Braz J Med Biol Res. 2001. 34: 475-8
205. Rijzewijk LJ, van der Meer RW, Lubberink M, Lamb HJ, Romijn JA, de Roos A. Liver fat content in type 2 diabetes: Relationship with hepatic perfusion and substrate metabolism. Diabetes. 2010. 59: 2747-54
206. Ritchie SA, Connell JM. The link between obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007. 17: 319-26
207. Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012. 287: 5301-9
208. Robert M, Ferrand-Gaillard C, Disse E, Espalieu P, Simon C, Laville M. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: Impact of surgical techniques. Obes Surg. 2013. 23: 770-5
209. Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999. 33: 548-53
210. Rocchini AP, Yang JQ, Gokee A. Hypertension and insulin resistance are not directly related in obese dogs. Hypertension. 2004. 43: 1011-6
211. Rodriguez L, Reyes E, Fagalde P, Oltra MS, Saba J, Aylwin CG. Pilot clinical study of an endoscopic removable duodenal jejunal bypass liner for treatment of type 2 diabetes. Diabetes Technol Ther. 2009. 11: 725-32
212. Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002. 236: 554-9
213. Rubino F, Kaplan LM, Schauer PR, Cummings DE. The diabetes surgery summit consensus conference: Recommendations for the gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010. 251: 399-405
214. Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: Clinical outcomes and mechanisms of action. Ann Rev Med. 2010. 61: 393-411
215. Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR. Neural mechanisms in human obesity related hypertension. J Hypertens. 1999. 17: 1125-33
216. Ruster C, Wolf G. The role of the renin angiotensin aldosterone system in obesity related renal diseases. Semin Nephrol. 2001. ;33: 44-53
217. Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory of venous drainage-Selective fat transplantation. Diabetes. 2011. 60: 56-63
218. Rzouq F, Alahdab F, Olyaee M. New insight into volume overload and hepatorenal syndrome in cirrhosis, “the hepatorenal reflex hypothesis”. Am J Med Sci. 2014. 348: 244-8
219. Sánchez-Lozada LG, Tapia E, Bautista-García P, Soto V, Avila-Casado C, Vega-Campos IP. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2008. 294: F710-8
220. Sandler BJ, Rumbaut R, Swain CP, Torres G, Morales L, Gonzales L. Human experience with an endoluminal endoscopic gastrojejunal bypass sleeve. Surg Endosc. 2011. 25: 3028-33
221. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G. Effect of laparoscopic Roux-en-Y on type 2 diabetes mellitus. Ann Surg. 2003. 238: 467-84
222. Schmid AI, Szendroedi J, Chmelik M, Krssák M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011. 34: 448-53
223. Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM. A multicenter, randomized efficacy study of the Endobarrier GI liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010. 251: 236-43
224. Scopinaro N, Adami GF, Papadia FS, Camerini G, Carlini F, Fried M. Effects of biliopancreatic diversion on type 2 diabetes in patients with BMI 25 to 35. Ann Surg. 2011. 253: 699-703
225. Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: A long-term follow-up study. Diabetes Care. 2005. 28: 2406-11
226. Seifalian AM, Chidambaram V, Rolles K, Davidson BR. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg. 1998. 4: 71-7
227. Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999. 68: 780-4
228. Seravalle G, Colombo M, Perego P, Giardini V, Volpe M, Dell’Oro R. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension. 2014. 64: 431-7
229. Serpa Neto A, Rossi FM, Valle LG, Teixeira GK, Rossi M. Relation of uric acid with components of metabolic syndrome before and after Roux-en-Y gastric bypass in morbidly obese subjects. Arq Bras Endocrinol Metabol. 2011. 55: 38-45
230. Shea JL, Randell EW, Sun G. The prevalence of metabolically healthy obese subjects defined by BMI and dual energy X-ray absorptiometry. Obesity. 2011. 19: 624-30
231. Shen D, Ye H, Wang Y, Ji Y, Zhan X, Zhu J. Comparison of short term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. Surg Endosc. 2013. 27: 2768-74
232. Sidery MB, Macdonald IA, Blackshaw PE. Superior mesenteric artery blood flow and gastric emptying in humans and the differential effects of high fat and high carbohydrate meals. Gut. 1994. 35: 186-90
233. Siregar H, Chou CC. Relative contribution of fat, protein and carbohydrate and ethanol to intestinal hyperemia. Am J Physiol. 1982. 242: G27-31
234. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial-A prospective controlled intervention study of bariatric surgery. J Intern Med. 2013. 273: 219-34
235. Snitker S, Macdonald I, Ravussin E, Astrup A. The sympathetic nervous system and obesity: Role in etiology and treatment. Obes Rev. 2000. 1: 5-15
236. Solis-Herruzo JA, Duran A, Favela V, Castellano G, Madrid JL, Muñoz-Yagüe MT. Effects of lumbar sympathetic block on kidney function in cirrhotic hepatorenal syndrome. J Hepatol. 1987. 5: 167-73
237. Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: Is it time for reappraisal?. Curr Hypertens Rep. 2013. 15: 175-81
238. Someya N, Endo MY, Fukuba Y, Hayashi N. Blood flow responses in celiac and superior mesenteric arteries in the initial phase of digestion. Am J Physiol Regul Integr Comp Physiol. 2008. 294: R1790-6
239. Soresi M, Giannitrapani L, Noto D, Terranova A, Campagna ME, Cefalù AB. Effects of steatosis on hepatic hemodynamics in patients with metabolic syndrome. Ultrasound Med Biol. 2015. 41: 1545-52
240. Sovik TT, Taha O, Aasheim ET, Engstrom M, Kristinsson J, Bjorkman S. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br Med J. 2009. 97: 160-6
241. Stadlbauer V, Wright GA, Banaji M, Mukhopadhya A, Mookerjee RP, Moore K. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008. 134: 111-9
242. Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. 2016. 53: 52-67
243. Stefater M, Kohli R, Inge TH. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013. 34: 84-94
244. Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: Insights from mechanistic comparisons. Endocr Rev. 2012. 33: 595-622
245. Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008. 10: 440-7
246. Straznicky NE, Grima MT, Lambert EA, Sari CI, Eikelis N, Nestel PJ. Arterial norepinephrine concentration is inversely and independently associated with insulin clearance in obese individuals with metabolic syndrome. J Clin Endocrinol Metab. 2015. 100: 1544-50
247. Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ. Blunted sympathetic neural response to oral glucose in obese subjects. Am J Clin Nutr. 2009. 89: 27-36
248. Stylopoulos N, Aguirre V. Mechanisms of bariatric surgery and implications for the development of endoluminal therapies for obesity. Gastrointest Endosc. 2009. 70: 1167-75
249. Sultan S, Gupta D, Parikh M, Youn H, Kurian M, Fielding G. Five year outcomes of patients with type 2 diabetes who underwent laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2010. 6: 373-6
250. Svane MS, Madsbad S. Bariatric surgery - effects on obesity and related co-morbidities. Curr Diabetes Rev. 2014. 10: 208-14
251. Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009. 50: 1079-86
252. Takala J. Determinants of splanchnic blood flow. Br J Anesth. 1996. 77: 50-8
253. Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010. 90: 23-46
254. Van den Berghe G, Bontemps F, Vincent MF. Cytosolic purine 5’- nucleotidases of rat liver and human red blood cells: Regulatory properties and role in AMP dephosphorylation. Adv Enzyme Regul. 1988. 27: 297-311
255. Van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977. 162: 601-9
256. Vatner SF, Franklin D, Van Citters RL. Mesenteric vasoactivity associated with eating and digestion in the conscious dog. Am J Physiol. 1970. 219: 170-4
257. Vaz M, Cox HS, Kaye DM, Turner AG, Jennings GL, Esler MD. Fallibility of plasma noradrenaline measurements in studying postprandial sympathetic nervous responses. J Auton Nerv Syst. 1995. 56: 97-104
258. Verna EC, Wagener G. Renal interactions in liver dysfunction and failure. Curr Opin Crit Care. 2013. 19: 133-41
259. Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: Effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009. 150: 94-103
260. Vidal P, Ramón JM, Goday A, Benaiges D, Trillo L, Parri A. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive procedure for morbid obesity. Mid-term results. Obes Surg. 2013. 23: 292-9
261. Vincent MF, Van den Berghe G, Hers HG. The pathway of adenine nucleotide catabolism and its control in isolated rat hepatocytes subjected to anoxia. Biochem J. 1982. 202: 117-23
262. Vollmar B, Menger MD. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009. 89: 1269-339
263. Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: Pathophysiology and management. Clin J Am Soc Nephrol. 2006. 1: 1066-79
264. Weidmann P, de Courten M, Boehlen L, Shaw S. The pathogenesis of hypertension in obese subjects. Drugs. 1993. 46: 197-209
265. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: A time course study. Obes Surg. 2005. 15: 474-81
266. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008. 168: 1617-24
267. Wilhelm SM, Young J, Kale-Pradhan PB. Effect of bariatric surgery on hypertension: A meta-analysis. Ann Pharmacother. 2014. 48: 674-82
268. Williams S, Cunningham E, Pories WJ. Surgical treatment of metabolic syndrome. Med Princ Pract. 2012. 21: 301-9
269. Yamaguchi N, Suzuki-Kusaba M, Hisa H, Hayashi Y, Yoshida M, Satoh S. Interaction between norepinephrine release and intrarenal angiotensin II formation during renal nerve stimulation in dogs. J Cardiovasc Pharmacol. 2000. 35: 831-7
270. Yu H, Zhang L, Bao Y, Zhang P, Tu Y, Di J. Metabolic Syndrome After Roux-en-Y Gastric Bypass Surgery in Chinese Obese Patients with Type 2 Diabetes. Obes Surg. 2016. p.
271. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH. HOPE Study Investigators. Ramipril and the development of diabetes. JAMA. 2001. 286: 1882-5
272. Zacho HD, Henriksen JH, Abrahamsen J. Chronic intestinal and splanchnic blood flow: Reference values and correlation with body-composition. World J Gastroenterol. 2013. 19: 882-8
273. Zhang C, Yuan Y, Qiu C, Zhang W. A meta-analysis of 2-year effect after surgery: Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus. Obes Surg. 2014. 24: 1528-35
274. Zhang H, Pu Y, Chen J, Tong W, Cui Y, Sun F. Gastrointestinal intervention ameliorates high blood pressure through antagonizing overdrive of the sympathetic nerve in hypertensive patients and rats. J Am Heart Assoc. 2014. 3: e000929-
275. Zhang N, Maffei A, Cerabona T, Pahuja A, Omana J, Kaul A. Reduction in obesity related comorbidities: Is gastric bypass better than sleeve gastrectomy. Surg Endosc. 2013. 27: 1273-80
276. Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity associated risk factors among whites. Am J Clin Nutr. 2002. 76: 743-9
277. Zimmerman MA, Kam I, Eltzschig H, Grenz A. Biological implications of extracellular adenosine in hepatic ischemia and reperfusion injury. Am J transplant. 2013. 13: 2524-9