- Department of Neurosurgery, University Hospital Centre Zagreb, Zagreb 10 000, Croatia
- School of Medicine, University of Zagreb, Zagreb 10 000, Croatia
Correspondence Address:
Kresimir Sasa Duric
Department of Neurosurgery, University Hospital Centre Zagreb, Zagreb 10 000, Croatia
DOI:10.4103/2152-7806.179571
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mrak G, Duric KS, Nemir J. Middle cerebral artery fusiform aneurysm presented with stroke and delayed subarachnoid hemorrhage trapping, thrombectomy, and bypass. Surg Neurol Int 01-Apr-2016;7:
How to cite this URL: Mrak G, Duric KS, Nemir J. Middle cerebral artery fusiform aneurysm presented with stroke and delayed subarachnoid hemorrhage trapping, thrombectomy, and bypass. Surg Neurol Int 01-Apr-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/middle-cerebral-artery-fusiform-aneurysm-presented-with-stroke-and-delayed-subarachnoid-hemorrhage-trapping-thrombectomy-and-bypass/
Abstract
Background:Ischemic stroke is a well-described but less frequent consequence of ruptured or unruptured intracranial aneurysms. To date, the optimal form of treatment for patients with a thrombosed cerebral aneurysm has not yet been well-defined.
Case Description:Here, we report a case of a 68-year-old female patient presenting with cerebral stroke. Five days poststroke multislice computed tomography (MSCT) and MSCT angiography were performed for the evaluation of clinical deterioration, showing a left M2 middle cerebral artery (MCA) bifurcation aneurysm and subarachnoid hemorrhage. Having in mind the high mortality and morbidity rates after a re-rupture, as well as the digital subtraction angiography features of the aneurysm, urgent surgery was performed consisting of aneurysm trapping and superficial temporal artery (STA) to M3 MCA segment end-to-side anastomosis. The surgery and early postoperative period proceeded uneventfully and the patient gradually recovered from the previously diagnosed expressive dysphasia and cranial and extremity motor deficit.
Conclusion:Our case describes a complex aneurysm treatment that consisted of aneurysm trapping, thrombus removal and an STA-M3 MCA branch bypass creation for the protection of the patent M3 insular MCA branch and prevention of further ischemia. This procedure rewarded us with an excellent clinical result.
Keywords: Cerebral ischemia, intracranial aneurysm, stroke
INTRODUCTION
Ischemic stroke is a well-described but less frequent consequence of ruptured or unruptured intracranial aneurysms. Thrombus formation linked to transient ischemic attacks (TIAs) and ischemic stroke has been reported in giant and fusiform as well as in small aneurysms. The reported prevalence rates of cerebral ischemia directly associated with intracranial aneurysm vary from 0.6% to 11%.[
We report a case of a 68-year-old female patient diagnosed with a cerebral stroke. Five days poststroke native head multislice computed tomography (MSCT) and MSCT angiography (MSCTA) were performed for the evaluation of clinical deterioration. Studies revealed a left M2 middle cerebral artery (MCA), bifurcation aneurysm, and subarachnoid hemorrhage (SAH). Surgical treatment consisted of aneurysm trapping and superficial temporal artery (STA) to M3 MCA segment end-to-side anastomosis.
CASE REPORT
Preoperative course
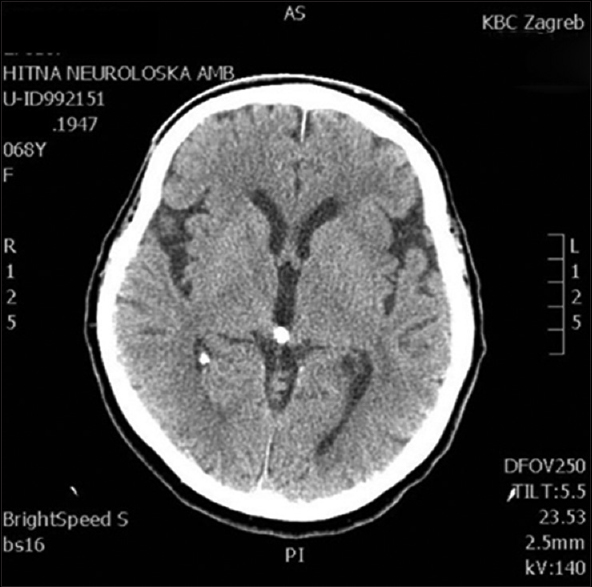
This 68-year-old female patient presented with right-sided supranuclear facial palsy and right-sided extremity palsy. The symptoms were of sudden onset with no accompanying headaches or vomiting, or a history of recent trauma. The initial head MSCT showed no obvious signs of cerebral ischemia [
The patient's medical history revealed chronic diseases which included arterial hypertension, diabetes mellitus, and chronic gastritis, as well as surgical and oncological treatment for an invasive intraductal breast carcinoma.
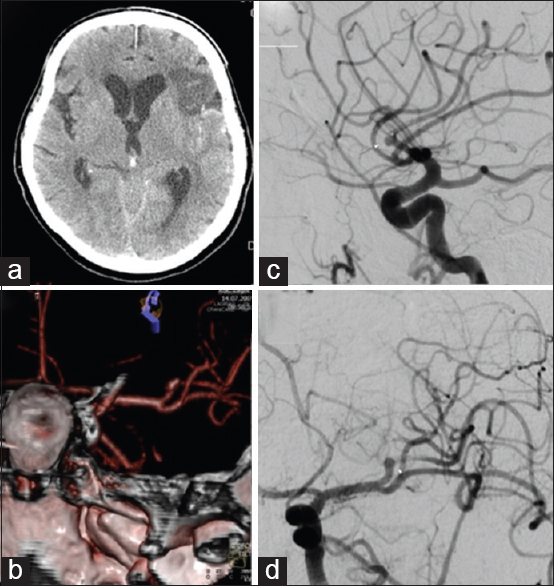
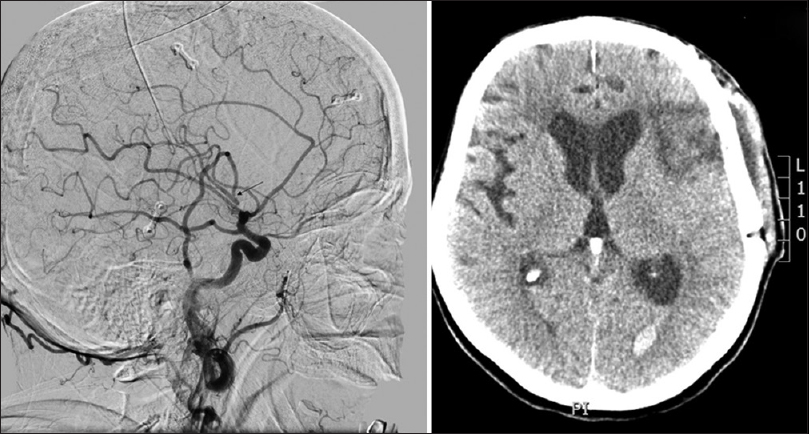
Four days after an uneventful stay at the Department of Neurology, the patient experienced a severe headache followed by vomiting and dysphasia, as well as deterioration of consciousness, thus becoming somnolent. Urgent MSCT revealed a demarcated ischemic area in the left frontal opercular region and an SAH in the left sylvian fissure, as well as the presence of blood in the IV ventricle. MSCTA and digital subtraction angiography (DSA) revealed an aneurysm on the left second M2 MCA branch bifurcation, 5 mm from the primary MCA bifurcation. The absence of blood flow was noticed in the “smaller” frontal opercular M3 segment, implying thrombosis. Filiform blood flow was noticed in the “larger” insular M3 segment, resembling partial vessel thrombosis. The aneurysm seemed to be fusiform, just at the second bifurcation, with full thrombosis of the frontal opercular branch. There were no radiological signs of vasospasm. By comparing the DSA and MSCT scans, it could be concluded that the fully thrombosed frontal M3 branch was the cause of the cerebral ischemia and that the second opercular M3 branch was in peril due to partial thrombosis [
Figure 2
(a) Native multislice computed tomography showing left-side fronto-opercular cerebral ischemia and subarachnoid hemorrhage. (b) Multislice computed tomography angiography showing an aneurysm on the left M2 middle cerebral artery branch 5 mm from the M1 middle cerebral artery branch bifurcation. Digital subtraction angiography, lateral (c) and anteroposterior (d) view showing an M2 branch bifurcation aneurysm, absence of blood flow in the “smaller” frontal opercular M3 segment implying thrombosis and filiform blood flow in the “larger” insular M3 segment resembling partial vessel thrombosis
The left P1 segment was hypoplastic, and the posterior cerebral arteries on both sides were dominantly supplied by the posterior communicating artery. The right vertebral artery showed to be hypoplastic. There were no signs of the presence of other saccular aneurysms or arteriovenous malformations.
As the shown aneurysm was favorable for surgical management, urgent surgery was indicated.
Operation
Surgery objectives
As DSA revealed a fusiform-shaped aneurysm at the left M2 MCA bifurcation on the superior M2 branch with signs of postaneurysm thrombosis, the plan was to prepare the STA for a possible bypass.
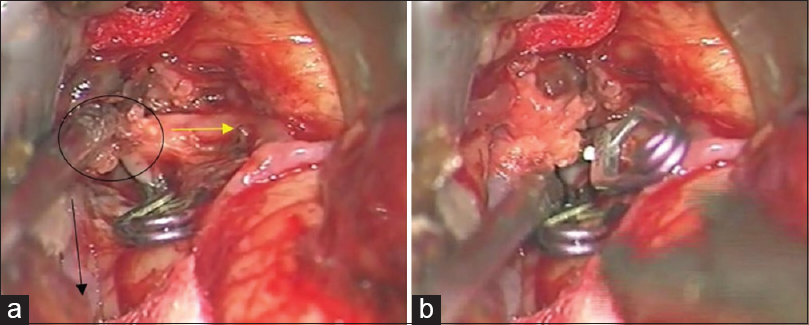
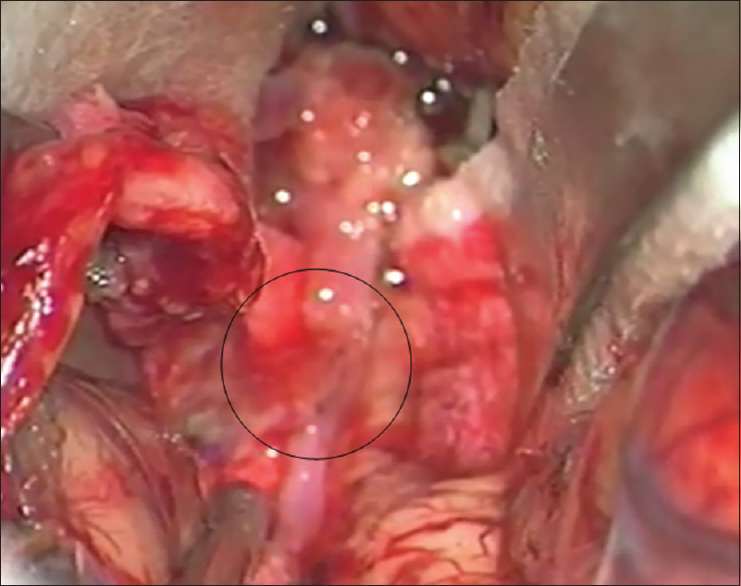
Left osteoplastic frontotemporal craniotomy with the preparation of the frontal STA branch was performed under general anesthesia and in the supine position, with the head rotated to the right and positioned in a Mayfield holder. After the removal of the bone, the dura was opened and suspended downward. The left sylvian fissure was widely opened and a fusiform aneurysm at the superior M2 bifurcation, including two M3 branches 5 mm from the M1 MCA segment bifurcation was intraoperatively verified. The flow in both M3 branches was checked by micro-Doppler, revealing the absence of flow and verifying a thrombosed frontal opercular M3 vessel irrigating the already ischemic part of the frontal operculum. The larger insular M3 MCA branch showed no flow on Doppler analysis, as compared to the visible filiform flow shown on the previous DSA. Bearing in mind the clearly ischemic portion of the frontal operculum irrigated by the smaller upper M3 branch, as shown by DSA and micro-Doppler, as well as the fact that the whole circumference of the vessel wall was evidently changed into a fusiform aneurysm, the permanent solution was complete M2 occlusion by placing a clip proximally to the aneurysm [
Figure 3
(a) Intraoperative picture showing fusiform aneurysm on the M2 middle cerebral artery branch, encircled. Smaller upper M3 branch is marked with a yellow arrow and lower larger M3 middle cerebral artery branch is marked with a black arrow. A temporary clip has been placed on the M2 while aneurysm preparation is performed. (b) Intraoperative picture showing definite clip placement on the M2 middle cerebral artery branch for definite occlusion
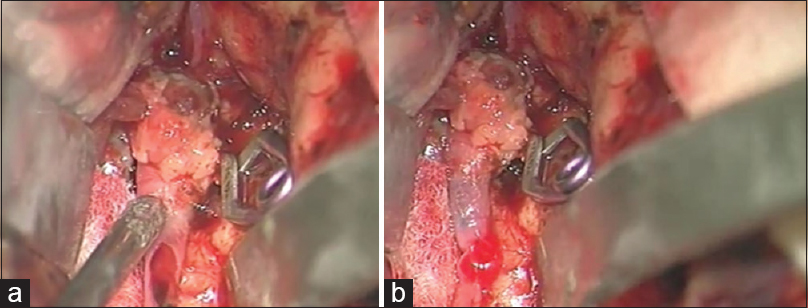
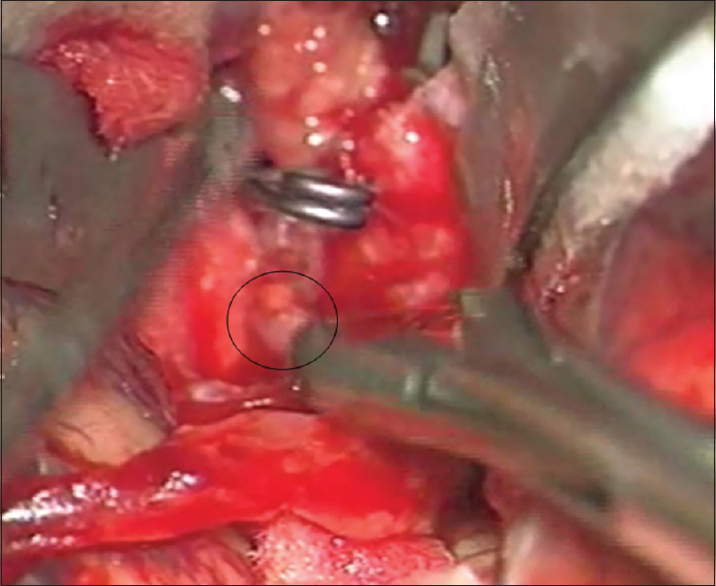
The postaneurysm part of the lower larger M3 MCA branch was opened with a small linear incision confirming the thrombus within it. After the thrombus evacuation and verified backflow, we decided to protect this vessel by creating an STA-MCA bypass [
As a preparation for the bypass procedure, the patient was given a loading dose of 300 mg of clopidogrel and 300 mg of aspirin. The STA was prepared with a fish-mouth opening and sutured with 10-0 prolene to the recipient vessel by end-to-side anastomosis [
After the bypass procedure, back-bleeding from the aneurysm occurred. This bleeding was successfully stopped by placing another clip distal to the aneurysm and in front of the bypass on the lower larger M3 MCA branch, thus creating a definite aneurysm trapping [
A second bypass or vessel occlusion by clipping was not performed on the upper smaller branch that irrigated the already ischemic part of the frontal operculum.
A micro Doppler was used to check the bypass patency, and upon patency confirmation, the wound was closed in the usual manner. A notch was created on the bone flap to protect the STA vessel.
Postoperative course
On the second postoperative day, DSA showed a patent bypass [
DISCUSSION
As shown in previous studies, TIAs and cerebral infarctions are associated with intracranial aneurysms. It is clear that an aneurysm sac can act as a source of distal embolization. Reported prevalence rates of cerebral ischemia directly linked to intracranial aneurysm vary from 0.6% to 11%.[
Nagashima et al. observed that surgical treatment of aneurysms in the presence of cerebral infarctions represented high-risk procedures, and because of the high morbidity and mortality rates in such cases they recommended a conservative approach for most of these patients.[
Aneurysm thromboses after SAH have been infrequently reported with an incidence of 1–2%.[
Today, in the advanced endovascular era, the need for intracranial bypass surgery has dramatically decreased, and it is only recommended for selected cases where endovascular intervention is not feasible. According to Kalani et al., the most frequent indications for intracranial bypass surgery in the modern era are complex aneurysms, moyamoya disease, and vascular occlusive disease.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Antunes JL, Correll JW. Cerebral emboli from intracranial aneurysms. Surg Neurol. 1976. 6: 7-10
2. Atkinson JL, Lane JI, Colbassani HJ, Llewellyn DM. Spontaneous thrombosis of posterior cerebral artery aneurysm with angiographic reappearance. Case report. J Neurosurg. 1993. 79: 434-7
3. Brownlee RD, Tranmer BI, Sevick RJ, Karmy G, Curry BJ. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm. An unusual cause of ischemic stroke. Stroke. 1995. 26: 1945-9
4. Crompton MR. The pathogenesis of cerebral infarction following the rupture of cerebral berry aneurysms. Brain. 1964. 87: 491-510
5. Edner G, Forster DM, Steiner L, Bergvall U. Spontaneous healing of intracranial aneurysms after subarachnoid hemorrhage. Case report. J Neurosurg. 1978. 48: 450-4
6. Eller TW. MRI demonstration of clot in a small unruptured aneurysm causing stroke. Case report. J Neurosurg. 1986. 65: 411-2
7. Fisher M, Davidson RI, Marcus EM. Transient focal cerebral ischemia as a presenting manifestation of unruptured cerebral aneurysms. Ann Neurol. 1980. 8: 367-72
8. Fodstad H, Liliequist B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid (AMCA). Report of three cases. Acta Neurochir (Wien). 1979. 49: 129-44
9. Friedman JA, Piepgras DG, Pichelmann MA, Hansen KK, Brown RD, Wiebers DO. Small cerebral aneurysms presenting with symptoms other than rupture. Neurology. 2001. 57: 1212-6
10. Fukuoka S, Suematsu K, Nakamura J, Matsuzaki T, Satoh S, Hashimoto I. Transient ischemic attacks caused by unruptured intracranial aneurysm. Surg Neurol. 1982. 17: 464-7
11. Hamilton MG, Dold ON. Spontaneous disappearance of an intracranial aneurysm after subarachnoid hemorrhage. Can J Neurol Sci. 1992. 19: 389-91
12. Kalani MY, Ramey W, Albuquerque FC, McDougall CG, Nakaji P, Zabramski JM. Revascularization and aneurysm surgery: Techniques, indications, and outcomes in the endovascular era. Neurosurgery. 2014. 74: 482-97
13. Kalani MY, Rangel-Castilla L, Ramey W, Nakaji P, Albuquerque FC, McDougall CG. Indications and results of direct cerebral revascularization in the modern era. World Neurosurg. 2015. 83: 345-50
14. Lin JP. Thrombosis of aneurysm of anterior communicating artery. Case report. Acta Radiol Diagn (Stockh). 1969. 8: 74-80
15. Nagashima M, Nemoto M, Hadeishi H, Suzuki A, Yasui N. Unruptured aneurysms associated with ischaemic cerebrovascular diseases. Surgical indication. Acta Neurochir (Wien). 1993. 124: 71-8
16. Nanda A, Vannemreddy PS. Cerebral ischemia as a presenting feature of intracranial aneurysms: A negative prognostic indicator in the management of aneurysms. Neurosurgery. 2006. 58: 831-7
17. Ohno K, Suzuki R, Masaoka H, Matsushima Y, Monma S, Inaba Y. Unruptured aneurysms in patients with transient ischemic attack or reversible ischemic neurological deficit. Report of eight cases. Clin Neurol Neurosurg. 1989. 91: 229-33
18. Okawara SH. Warning signs prior to rupture of an intracranial aneurysm. J Neurosurg. 1973. 38: 575-80
19. Parenti G, Fiori L, Marconi F. Intracranical aneurysm and cerebral embolism. Eur Neurol. 1992. 32: 212-5
20. Qureshi AI, Mohammad Y, Yahia AM, Luft AR, Sharma M, Tamargo RJ. Ischemic events associated with unruptured intracranial aneurysms: Multicenter clinical study and review of the literature. Neurosurgery. 2000. 46: 282-9
21. Sakaki T, Kinugawa K, Tanigake T, Miyamoto S, Kyoi K, Utsumi S. Embolism from intracranial aneurysms. J Neurosurg. 1980. 53: 300-4
22. Shah S, Murthy SB, Hannawi Y, Rao CP. The utility of cone beam volume CT in the evaluation of thrombosed intracranial aneurysms in subarachnoid hemorrhage. J Neurointerv Surg. 2013. 5: e51-
23. Spallone A, Peresedov VV, Kandel EI. Spontaneous cure of ruptured intracranial arterial aneurysms. Surg Neurol. 1981. 16: 367-70
24. Sutherland GR, King ME, Peerless SJ, Vezina WC, Brown GW, Chamberlain MJ. Platelet interaction within giant intracranial aneurysms. J Neurosurg. 1982. 56: 53-61
25. Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982. 45: 1040-7