- Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
DOI:10.4103/2152-7806.182415
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hansen D, Kan PT, Reddy GD, Mohan AC, Jea A, Lam S. Pediatric knowledge update: Approach to the management of vein of Galen aneurysmal malformations in neonates. Surg Neurol Int 13-May-2016;7:
How to cite this URL: Hansen D, Kan PT, Reddy GD, Mohan AC, Jea A, Lam S. Pediatric knowledge update: Approach to the management of vein of Galen aneurysmal malformations in neonates. Surg Neurol Int 13-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/pediatric-knowledge-update-approach-to-the-management-of-vein-of-galen-aneurysmal-malformations-in-neonates/
Abstract
Keywords: Neonate, pediatric, vascular, vein of Galen aneurysmal malformation, vein of Galen malformation
CASE EXAMPLE
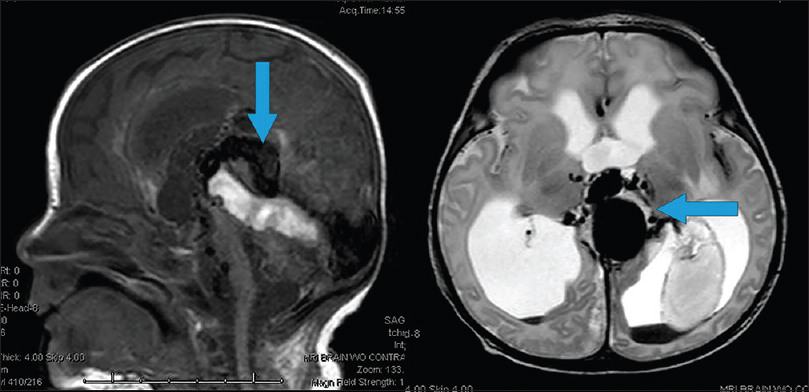
A newborn female born via cesarean section at 39 weeks gestation was transferred to our institution for severe respiratory distress after delivery. She was intubated and required 100% fraction of inspired oxygen to maintain adequate saturations. She was also started on vasopressor medications secondary to hypotension. An echocardiogram of the heart showed diastolic flow reversal in the transverse aortic arch and increased flow in the superior vena cava. A bruit was auscultated over the anterior fontanelle that was concerning for an intracranial vascular malformation. Bedside, head ultrasound confirmed the presence of a vein of Galen malformation. Her liver was enlarged without signs of liver failure. Her anuria resolved with improved renal function after her hypotension was treated. Electroencephalography was negative for seizure activity. Her Bicêtre score was calculated to be between 9 and 11. As per the Lasjaunias algorithm, the baby was a candidate for emergent endovascular embolization.
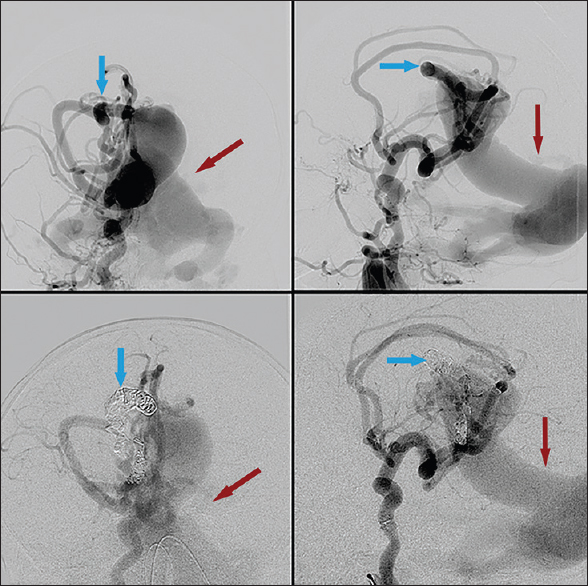
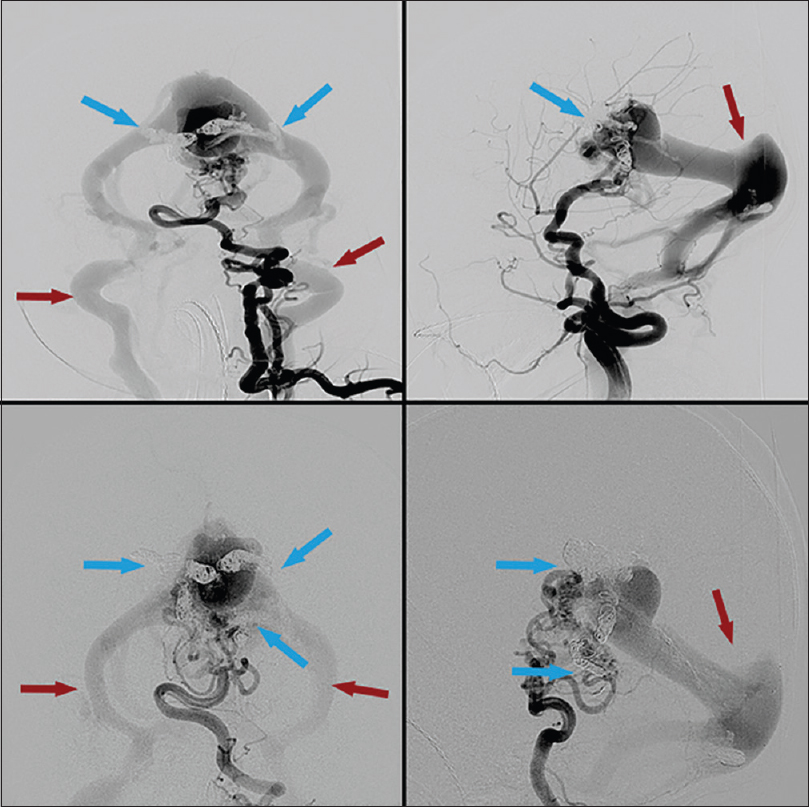
Cerebral angiogram revealed an extensive choroidal type vein of Galen malformation with severe arteriovenous shunting [Figures
Figure 1
First embolization treatment. Right internal carotid injection. Pretreatment (top) and posttreatment (bottom). Blue arrows highlighting arterial feeders pre- and post-coil embolization. Note slightly decreased contrast filling of venous outflow after coil placement, highlighted by red arrows
BACKGROUND
Neonatal vein of Galen aneurysmal malformations (VGAMs) are rare; the incidence is approximately 1 in 25,000.[
VGAM represents an embryonic arteriovenous shunt in the subarachnoid space and the choroidal fissure. Steinheil first described this entity in 1895, but it was Boldrey and Miller in 1949 who first documented a true form of VGAM by identifying multiple arteriovenous communications draining into the dilated vein of Galen.[
The diagnosis can be made in utero by ultrasound and can be identified between the 6th and 11th week of gestation. Fetal magnetic resonance imaging can better evaluate cerebral atrophy, ventricular size, and cardiac insufficiency. Fetal distress presents as cardiac insufficiency and/or hydrops. Neonatal diagnosis is typically made after identifying severe cardiopulmonary distress during or acutely after birth, along with the associated pulmonary, neurological, hepatic, and renal dysfunction.[
Before the advent of endovascular interventions, mortality in neonatal VGAM was nearly 90%.[
In 2006, Lasjaunias described his large experience with VGAM management. The primary and immediate goal is stabilization of the life-threatening congestive heart failure. The next step is to attempt treatment of the VGAM. He recommends a staged approach to better manage the risk that comes with the instant flow reversal with obliteration of the VGAM. Any presenting hydrocephalus is not treated until after treatment of the VGAM to avoid worsening flow through the VGAM, which can occur with lowering of intracranial pressure.[
EVALUATION AND TREATMENT
Initial consultation should include a thorough neurological examination, bedside electroencephalography, auscultation for cranial bruit, measurement of head circumference, and signs or symptoms of hydrocephalus. Although the immediate clinical concern is cardiac failure, VGAM has direct cerebral consequences including encephalopathy, hydrocephalus, seizures, and developmental delay.
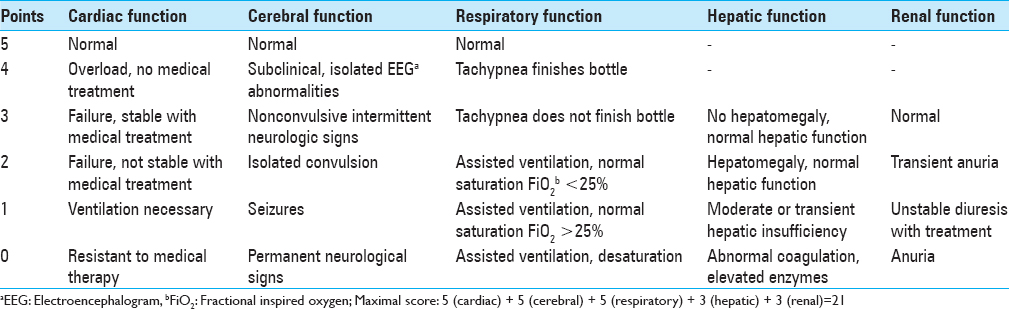
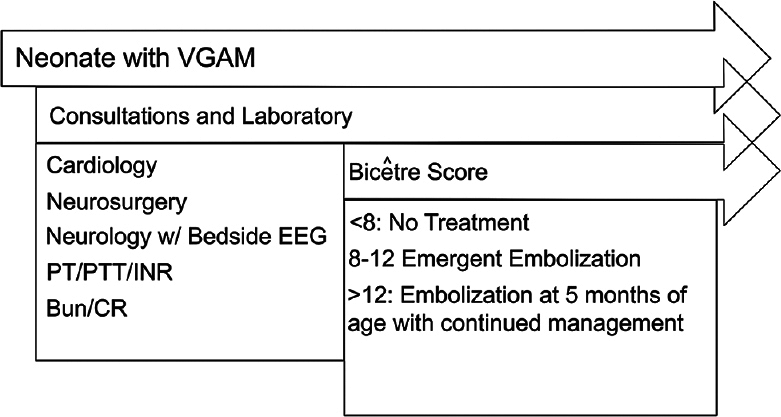
One option for evaluation of the newborn with VGAM is based on the Bicκtre score to determine potential treatment options.[
Cardiology assessment of cardiac function and superior vena cava flow should proceed right away. An echocardiogram should be performed to assess for the function of all the cardiac chambers, the presence of a pulmonary ductus arteriosus shunt, degree of pulmonary arterial pressure (measured indirectly with the velocity of flow through the insufficient tricuspid valve), and other cardiac anomalies such as atrial septal defect and aortic coarctation.
Potential medical treatments are aimed at controlling the heart's left-to-right shunting caused by the VGAM. Nitric oxide can aid with persistent pulmonary hypertension. Other pharmacologic agents include diuretics and beta agonists such as dopamine, inotropes, prostaglandins, or phosphodiesterase inhibitors.[
Laboratory tests should evaluate liver and renal function, in addition to serving as secondary markers of cardiac and pulmonary status. Brain natriuretic protein levels act as a marker for cardiac injury due to pulmonary hypertension.[
TREATMENT
Treatment options for VGAMs have historically included either open surgery, a direct transtorcular approach to ligation, transarterial embolization, or transvenous embolization. Reports show nearly 100% mortality with open surgical approaches; transtorcular approaches have, likewise, not been successful; and transvenous approaches are reported to have poor cognitive outcomes.[
Transarterial embolization, in a staged fashion, for partial occlusion of the VGAM is the only current treatment that has been shown to result in a safe reduction of cardiac failure. Access in neonates is often found through the umbilical artery. Protecting this route of access during birth and the acute postnatal period is important. This artery has the benefit of providing adequate access for the needed endovascular catheters, while sparing trauma to the femoral artery in small neonates. It is essential to maintain the patency of this vessel and utilize other routes for serial blood work.[
Embolization aims to occlude, or partially occlude, the arterial side of the fistula. Currently, there are two primary materials used: (1) N-butyl-cyanoacrylate (NBCA) or (2) Onyx liquid, an ethylene vinyl alcohol copolymer that is delivered in dimethyl sulfoxide (DMSO) solvent. Once in contact with an ionic substance (blood), the DMSO quickly dissolves, leaving a firm spongy substance that rapidly occludes the vessel. It is admixed with tantalum powder to render it visible on fluoroscopy and computed tomography. This gives it a dark purple/black appearance in vivo. The use of Onyx has been largely described in the adult population, but there is a growing body of literature supporting its use as safe in pediatric patients when used by experienced practitioners.[
The extreme flow through the VGAM is a major concern during endovascular treatment. Injection of the embolic material is difficult to control at such high flow rates and can lead to the NBCA or Onyx passing through the fistula without successfully depositing in the VGAM. There are three general techniques to deal with this problem.First, coils can be placed distally in the arterial pedicle to catch the Onyx or NBCA.[
After treating the primary problem of the VGAM, there may still be associated hydrocephalus to be addressed. Diagnosis is made by a combination of anterior fontanelle evaluation, head circumference measurements, and ultrasound assessment of ventricular caliber; more advanced imaging modalities may be used as well. The purported causes for the development of hydrocephalus in this setting include ineffective cerebrospinal fluid (CSF) reabsorption due to venous stenosis outside the VGAM, underdeveloped arachnoid granulations, and aqueductal obstruction resulting from the mass effect of the VGAM. Treatment should not precede control of the cardiac failure and VGAM.[
OUTCOMES
VGAM treated in neonates carries significant risks. The largest series from Lasjaunias at the Hospital Bicêtre determined that of the 216 patients treated with endovascular embolization, 23 (10.6%) died, 20 (10.5%) suffered severe intellectual disabilities, and 30 (15.6%) were moderately intellectually disabled. The remaining 143 (74%) were neurologically normal at follow-up.[
The management of VGAM is evolving, and the addition of transarterial embolization techniques is allowing increased treatment of these complex lesions with improving outcomes. Nevertheless, overall prognosis remains poor. Even with recent technological advances, a large population of patients remains untreatable or has poor long-term outcomes. Moving forward, there is a need for prospective studies, clinical registries, and ideally, a randomized controlled trial to determine the best medical and interventional treatments in these complex neonates.
At our institution, our collaborative multidisciplinary team follows the diagnostic and treatment algorithm outlined in
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alvarez H, Garcia Monaco R, Rodesch G, Sachet M, Krings T, Lasjaunias P. Vein of Galen aneurysmal malformations. Neuroimaging Clin N Am. 2007. 17: 189-206
2. Ashour R, Aziz-Sultan MA, Soltanolkotabi M, Schoeneman SE, Alden TD, Hurley MC. Safety and efficacy of Onyx embolization for pediatric cranial and spinal vascular lesions and tumors. Neurosurgery. 2012. 71: 773-84
3. Berenstein A, Fifi JT, Niimi Y, Presti S, Ortiz R, Ghatan S. Vein of Galen malformations in neonates: New management paradigms for improving outcomes. Neurosurgery. 2012. 70: 1207-13
4. Chevret L, Durand P, Alvarez H, Lambert V, Caeymax L, Rodesch G. Severe cardiac failure in newborns with VGAM.Prognosis significance of hemodynamic parameters in neonates presenting with severe heart failure owing to vein of Galen arteriovenous malformation. Intensive Care Med. 2002. 28: 1126-30
5. Foran A, Donohue V, McParland P, Lynch B, Lasjaunias P, Rodesch G. Vein of Galen aneurysm malformation (VGAM): Closing the management loop. Ir Med J. 2004. 97: 8-10
6. Frawley GP, Dargaville PA, Mitchell PJ, Tress BM, Loughnan P. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch Dis Child Fetal Neonatal Ed. 2002. 87: F144-9
7. Fullerton HJ, Aminoff AR, Ferriero DM, Gupta N, Dowd CF. Neurodevelopmental outcome after endovascular treatment of vein of Galen malformations. Neurology. 2003. 61: 1386-90
8. Gailloud P, O’riordan DP, Burger I, Lehmann CU. Confirmation of communication between deep venous drainage and the vein of Galen after treatment of a vein of Galen aneurysmal malformation in an infant presenting with severe pulmonary hypertension. AJNR Am J Neuroradiol. 2006. 27: 317-20
9. Gailloud P, O’Riordan DP, Burger I, Levrier O, Jallo G, Tamargo RJ. Diagnosis and management of vein of Galen aneurysmal malformations. J Perinatol. 2005. 25: 542-51
10. Gold A, Ransohoff J, Carter S. Vein of Galen malformation. Acta Neurol Scand Suppl. 1964. 40: S1-31
11. Gupta AK, Varma DR. Vein of Galen malformations: Review. Neurol India. 2004. 52: 43-53
12. Iizuka Y, Kakihara T, Suzuki M, Komura S, Azusawa H. Endovascular remodeling technique for vein of Galen aneurysmal malformations – Angiographic confirmation of a connection between the median prosencephalic vein and the deep venous system. J Neurosurg Pediatr. 2008. 1: 75-8
13. Jea A, Bradshaw TJ, Whitehead WE, Curry DJ, Dauser RC, Luerssen TG. The high risks of ventriculoperitoneal shunt procedures for hydrocephalus associated with vein of Galen malformations in childhood: Case report and literature review. Pediatr Neurosurg. 2010. 46: 141-5
14. Jones BV, Ball WS, Tomsick TA, Millard J, Crone KR. Vein of Galen aneurysmal malformation: Diagnosis and treatment of 13 children with extended clinical follow-up. AJNR Am J Neuroradiol. 2002. 23: 1717-24
15. Khullar D, Andeejani AM, Bulsara KR. Evolution of treatment options for vein of Galen malformations. J Neurosurg Pediatr. 2010. 6: 444-51
16. Lasjaunias PL, Chng SM, Sachet M, Alvarez H, Rodesch G, Garcia-Monaco R. The management of vein of Galen aneurysmal malformations. Neurosurgery. 2006. 59: S184-94
17. Li AH, Armstrong D, terBrugge KG. Endovascular treatment of vein of Galen aneurysmal malformation: Management strategy and 21-year experience in Toronto. J Neurosurg Pediatr. 2011. 7: 3-10
18. Li MH, Li WB, Fang C, Gao BL. Transarterial embolization with berenstein liquid coils and N-butyl cyanoacrylate in a vein of Galen aneurysmal malformation: A case report. Korean J Radiol. 2007. 8: 164-8
19. Malhotra RK, Florez L, White D, Papasozomenos S, Covinsky M, Bhattacharjee M. Vein of Galen aneurysmal malformation associated with high output cardiac failure in three neonates. J Neuropathol Exp Neurol. 2007. 66: 438-
20. Pop R, Manisor M, Wolff V, Kehrli P, Marescaux C, Beaujeux R. Flow control using Scepter™ balloons for Onyx embolization of a vein of Galen aneurysmal malformation. Childs Nerv Syst. 2015. 31: 135-40
21. Schneider SJ, Wisoff JS, Epstein FJ. Complications of ventriculoperitoneal shunt procedures or hydrocephalus associated with vein of Galen malformations in childhood. Neurosurgery. 1992. 30: 706-8
22. Stephan S, Rodesch G, Elolf E, Wiemann D, Jorch G. Vein of Galen aneurysmal malformations: An ultrasonographic incidental finding – A case report. Case Rep Pediatr 2012. 2012. p.
23. Thiex R, Williams A, Smith E, Scott RM, Orbach DB. The use of Onyx for embolization of central nervous system arteriovenous lesions in pediatric patients. AJNR Am J Neuroradiol. 2010. 31: 112-20
24. Zaidi HA, Kalani MY, Spetzler RF, McDougall CG, Albuquerque FC. Multimodal treatment strategies for complex pediatric cerebral arteriovenous fistulas: Contemporary case series at Barrow Neurological Institute. J Neurosurg Pediatr. 2015. 15: 615-24