- Department of Anesthesia/ICU and Perioperative Medicine, Hamad Medical Corporation, Doha, Qatar
- Department of Neurosurgery Section, Department of Neurosciences, Hamad Medical Corporation, Doha, Qatar
Correspondence Address:
Nissar Shaikh
Department of Neurosurgery Section, Department of Neurosciences, Hamad Medical Corporation, Doha, Qatar
DOI:10.4103/2152-7806.166180
Copyright: © 2015 Mehesry TH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Mehesry TH, Shaikh N, Malmstrom MF, E. Marcus MA, Khan A. Ruptured spinal arteriovenous malformation: Presenting as stunned myocardium and neurogenic shock. Surg Neurol Int 28-Sep-2015;6:
How to cite this URL: Mehesry TH, Shaikh N, Malmstrom MF, E. Marcus MA, Khan A. Ruptured spinal arteriovenous malformation: Presenting as stunned myocardium and neurogenic shock. Surg Neurol Int 28-Sep-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/ruptured-spinal-arteriovenous-malformation-presenting-as/
Abstract
Background:Neurogenic pulmonary edema (NPE) is a clinical syndrome usually defined as an acute pulmonary edema occurring shortly after a central neurologic insult. NPE was identified 100 years ago, but it is still underappreciated in the clinical setup. NPE usually appears within minutes to hours after the injury. It has a high mortality rate if not recognized early and treated appropriately. Similarly, neurogenic shock is a known complication of spinal cord injury reported incidence is more than 20% in isolated upper cervical spinal injury. But NPE is rare to occur, and stunned myocardium (SM) is not reported in spinal arteriovenous malformation (AVM) rupture. SM is a reversible cardiomyopathy resulting in transient left ventricular dysfunction which has been described to occur in the setting of catecholamine release during situations of physiologic stress. We report a case of high spinal AVM rupture presenting as SM, NPE, and neurogenic shock.
Case Description:A 32-year-old male who presented with sudden onset of pain and weakness in upper limbs. Imaging studies showed AVM rupture by imaging techniques. Initially, the patient had severe hypertension, respiratory distress requiring intubation and ventilation, then he developed hypotension, bradycardia, and asystole, which required immediate cardiopulmonary resuscitation and atropine. He remained with quadriplegia and suffered from frequent episodes of bradycardia and asystole.
Conclusions:Spinal AVM rupture can present as neurogenic shock, stunned myocardium, and pulmonary edema. Early recognition of AVM rupture and prompt surgical intervention, as well as aggressive treatment of shock, may enhance recovery and decrease the long-term morbidity.
Keywords: Neurogenic pulmonary edema, neurogenic shock, spinal arteriovenous malformation rupture
INTRODUCTION
Hypotension with bradycardia caused by a spinal cord injury (SCI) is given the name of “neurogenic shock.” Bradycardia and cardiac arrest is well-known complication of cervical spine injury due to reduced sympathetic activity, which usually settles down within 6 weeks of injury.[
Immediately with the sudden rupture of an arteriovenous malformation (AVM) occurs release of huge amount of catecholamine which causes stunned myocardium (SM). Neurogenic SM (NSM) has most recently been explained by the “catecholamine hypothesis,” which describes the underlying activation of sympathetic nervous system activity as the cause of reversible left ventricular (LV) dysfunction. Specifically, catecholamine release from sympathetic nerves innervating the myocardium leads to the development of reversible wall motion abnormalities, typically presenting as global hypokinesia and occurring with mild troponin elevations. The hypotension that results from neurogenic shock places patients at increased risk of secondary spinal cord ischemia due to impairment of autoregulation. Association of neurogenic pulmonary edema (NPE), SM, and neurogenic shock with spontaneous rupture of spinal AVM has never been reported in the literature. There are few reports of NPE and SM as an initial complication of central nervous system insult. Spontaneous spinal cord hemorrhage causing SM and pulmonary edema as a neurogenic shock requiring cardiac pacing is an unusual presentation of this case.
CASE REPORT
A 33-year-old Indian male developed sudden upper limbs weakness with neck pain while talking to his family on the phone. Emergency medical services (EMS) were called by one of his friends. On arrival of EMS, Glasgow coma score (GCS) dropped to 10/15 and oxygen saturation (SpO2) 82%. On the way to hospital, GCS dropped to 3/15, blood pressure (BP) was 198/110, and he was intubated by EMS.
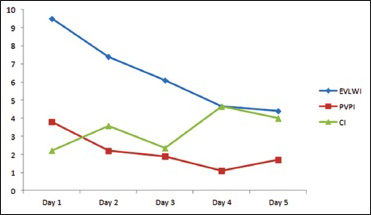
On arrival to emergency, BP dropped to 60/40 and heart rate (HR) 38 SpO2 85%, he received atropine 0.5 mg and was started on dopamine 5 mcg/kg/min. His blood gases were pH 7.155, PO2 61.2, PCO2 53.2, HCO3 18. Patient vitals stabilized BP 104/70 h 85 and SpO2 98%. Computed tomography scan showed evidence of linear hypodense area noted in the visualized portion of the upper cervical spine. Chest X-ray showed bilateral diffuse infiltrates suggestive of early signs of pulmonary edema. The patient suddenly became bradycardic HR 36 followed by asystole which was revived by atropine and cardiopulmonary resuscitation (CPR) for 4 min according to ACLS guidelines. He was sent to the intensive care. Upon arrival at the Intensive Care Unit (ICU), he was resuscitated with fluids and central venous catheters line and pulse induced continuous cardiac output (PiCCO) line inserted. He had another episode of bradycardia HR 30 followed by asystole, being stabilized after atropine 3 mg and 4 min of CPR. Neurological assessment by GCS on admission was 3/15 (E1, M1, VT), pupils right 4 mm, left 5 mm, and reacting to light. The patient had no cardiac problems in the past and his electrocardiogram following both the episodes showed no abnormalities other than sinus bradycardia. Aminophylline, dopamine 5–10 mcgkgmin, dobutamine 2.5 mcgkgmin intravenous infusions were started. PiCCO® study results confirmed pulmonary edema and low cardiac index [
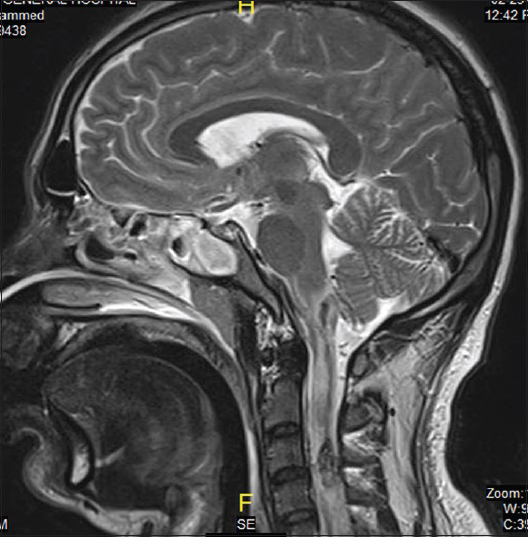
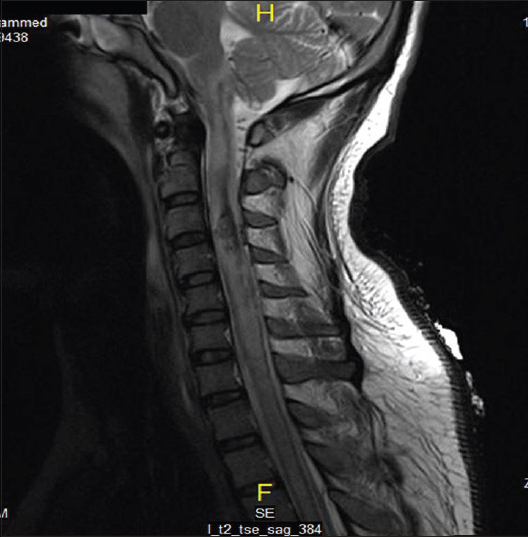
On the same day, echocardiogram was done which showed diffuse hypokinesia, mild impairment of left ventricle with apical wall motion abnormality ejection fraction (EF) of 40–45%, right ventricle systolic pressure (RVSP) of 41 mmHg. On the 2nd day, the patient was spontaneously opening eyes but complete quadriplegic though he was able to communicate with eyes and lips movements. It was documented as incomplete SCI as perianal sensation was intact. Magnetic resonance imaging study done showed sized area of hemorrhaging within the cervical cord extending from C1 to C4 secondary to rupture AVM [Figures
DISCUSSIONS
Our patient suffered insult in the upper cervical region of the spinal cord. We presumed that his hemodynamic instability is due to neurogenic shock, and further investigations showed the mild elevation of troponins with LV dysfunction which can be likely explained as NSM as the patient have no cardiac history before.
Neurogenic shock is almost always documented after an injury to the spinal cord.[
Hypotension with bradycardia caused by an SCI is named “neurogenic shock.” Neurogenic shock is most common when the level of the injury is above T6. It can happen as an initial presentation or may be weeks after the initial insult. The incidence of neurogenic shock in children with SCI is unknown. However, reports indicate anywhere from 50% to 90% of adults with cervical SCI require fluid resuscitation and vasoactive infusions to achieve the adult parameters recommended (mean arterial pressure >85–90 mmHg for 7 days) by the Congress of Neurological Surgeons’ guidelines for management of SCI. Adults with higher SCI (C1–C5) may be more likely to require cardiovascular interventions, such as vasoactive agents or cardiac pacing.[
Sympathetic innervation to heart arises from neurons in intermediolateral gray columns of cord segments T1–T4 which is under the control of higher centers via cervical spinal cord. Parasympathetic innervation comes via vagus nerve. Following any SCI above T1, the supraspinal control of sympathetic nervous system is lost, making the functioning spinal cord below the lesion independent of higher centers (known as “decentralization” of sympathetic nervous system), resulting in diminished sympathetic activity, while the parasympathetic control remains intact via vagus nerve, which results in relative parasympathetic dominance causing bradycardia and rarely cardiac arrest.[
This bradycardia will respond to atropine, glycopyrrolate, or vasoactive infusions with chronotropic, vasoconstrictor, and inotropic properties such as dopamine or noradrenaline.[
In 1908, Shanahan reported cases of acute pulmonary edema as a complication of epileptic seizures. Localized ischemic insult in suspected brain trigger zones (vasomotor centers, pulmonary input and output locations: Medulla oblongata, area postrema, caudal medulla, solitarius tractus nuclei) has been explained as one of the pathophysiological causes. Several clinicopathologic paradigms have been proposed to explain the clinical syndrome of NPE: (1) Neuro-cardiac; (2) neuro-hemodynamic; (3) “blast theory;” and (4) pulmonary venule adrenergic hypersensitivity.[
The release of large amounts of catecholamine causes severe systemic vasoconstriction and concomitantly, reduced LV diastolic and systolic compliance leads to an increased ventricular volume and extremely increasing the LV workload leading to SM.[
NPE though uncommon can occur in patients with spinal cord injuries and an important co-factor of morbidity and mortality. There is evidence that the motor cortex is involved in cardiovascular adjustments associated with somatic motor activity, as it has functional connections with the caudal ventrolateral (CVL) medulla, a brainstem region critically involved in the control of BP and the regulation of plasma catecholamine levels. The CVL medulla CVL sends projections to the spinal intermediolateral nucleus, where preganglionic neurons take control of heart and blood vessels (T2 segment) and adrenal medulla (T8 segment). Injury to the middle thoracic spine cord or upper level can probably justify the occurrence of hemodynamic disturbances.[
Focusing on our case, as the patient had no previous cardiac abnormalities, sudden onset of neurological problems, typical initial severe hypertension, respiratory distress, and postarrest finding of diffuse hypokinesia with EF 40% and RVSP of 40 mmHg in combination PiCCO findings supports the diagnosis of NPE and SM. A reduced EF and SM have both been described in association with NPE.
CONCLUSION
Cervical AVM rupture despite being rare can present with neurogenic shock, NPE, and SM. Early recognition of AVM rupture and prompt surgical intervention, as well as aggressive treatment of shock, may enhance recovery and decrease the long-term morbidity. Cardiac pacing for long-term patient safety should be considered in such cases.
Ruptured cervical AVM reported to present as chest pain and intracranial subarachnoid hemorrhage.[
References
1. Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 2007. 51: 447-55
2. Chen CC, Wang CM, Chu NK, Wu KP, Tang SF, Wong AM. Spinal cord arteriovenous malformation presenting as chest pain in a child. Spinal Cord. 2008. 46: 456-8
3. Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012. 16: 212-
4. Guly HR, Bouamra O, Lecky FE. Trauma Audit and Research Network. The incidence of neurogenic shock in patients with isolated spinal cord injury in the emergency department. Resuscitation. 2008. 76: 57-62
5. Sanghvi AV, Chhabra HS, Nigam V, Tandon V, Mascarenhas AA. Permanent cardiac pacemaker for cardiac arrest following cervico-dorsal spinal injury. Eur Spine J. 2009. 18: S254-7
6. Shaikh N, Raza A, Rahman A, Sabana A, Malmstrom F, Al Sulaiti G. prolonged bradycardia, asystole and outcome of high spinal cord injury patients. Panam J Trauma Crit Care Emerg Surg. 2014. 3: 87-92
7. van Beijnum J, Straver DC, Rinkel GJ, Klijn CJ. Spinal arteriovenous shunts presenting as intracranial subarachnoid haemorrhage. J Neurol. 2007. 254: 1044-51