- Department of Neurosurgery, Polytechnic University, Ancona, Marche, Italy
- Department of Plastic and Reconstructive Surgery, Operative Unit of Hand Surgery and Microsurgery, Polytechnic University, Ancona, Marche, Italy
Correspondence Address:
Alessandro Di Rienzo
Department of Neurosurgery, Polytechnic University, Ancona, Marche, Italy
DOI:10.4103/2152-7806.193724
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rienzo AD, Pangrazi PP, Riccio M, Colasanti R, Ghetti I, Iacoangeli M. Skin flap complications after decompressive craniectomy and cranioplasty: Proposal of classification and treatment options. Surg Neurol Int 09-Nov-2016;7:
How to cite this URL: Rienzo AD, Pangrazi PP, Riccio M, Colasanti R, Ghetti I, Iacoangeli M. Skin flap complications after decompressive craniectomy and cranioplasty: Proposal of classification and treatment options. Surg Neurol Int 09-Nov-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/skin-flap-complications-decompressive-craniectomy-cranioplasty-proposal-classification-treatment-options/
Abstract
Background:The list of complications reported after decompressive craniectomy (DC) and cranioplasty is progressively increasing. Nonetheless, the exact incidence of these events is still ill-defined. Problems affecting skin flaps after DC and cranioplasty have never been accurately analyzed in papers and their impact on patients’ prognosis is largely underestimated.
Methods:In a 10-year time, we treated by DC 450 patients, 344 of whom underwent cranioplasty, either with autologous bone or artificial implants (hydroxyapatite, polyetheretherketone, titanium, polymethylmethacrylate). Complications involving skin flaps and requiring re-surgery were observed and treated in 38 cases. We classified three main types of lesions: (1) dehiscence, (2) ulcer, and (3) necrosis. In all cases surgical decision making was performed in cooperation with plastic surgeons, to select the best treatment option.
Results:Dehiscence was reported in 28 cases, ulcer in 6, and necrosis in 4. Surgeries included flap re-opening and re-suturing, Z-plasty, rotational, advancement, or free flaps. Treatment complications required further surgical procedures in six patients.
Conclusions:In our experience, complications involving skin flaps after DC and post-DC cranioplasty cannot be considered a minor event because of their potential to further compromise the yet fragile conditions of these patients. Their management is complex and requires a multidisciplinary approach to get the better results.
Keywords: Cranioplasty, decompressive craniectomy, dermal graft, free flap, necrosis, skin flap
INTRODUCTION
In last years, some reports focused attention on complications occurring after decompressive craniectomy (DC) and postdecompressive cranioplasty.[
Despite completed and ongoing trials, until now the only certainty about DC relies on its capacity of restoring normal levels of intracranial pressure (ICP), which, according to detractors, seems to result in an enhanced survival with no improvement of prognosis. With the increasing experience, the list of complications affecting patients undergoing DC and post-DC cranioplasty is growing up.[
Starting from the above considerations, the small interest for troubles affecting skin flaps overlying decompressed brains comes as no surprise. Neurosurgeons’ expertise in this field may be considered, at least, “inadequate.” Moreover, traditional beliefs, including the idea that when a cranioplasty gets exposed, it must absolutely be removed, should be re-discussed, in light of the large availability of powerful antibiotics, the improvement of surgical techniques and the introduction of preformed implants realized in re-sterilizable materials. Here, we present our 10-year experience in this field, result of a continuous side-to-side collaboration with plastic surgeons. Relying on the complete lack of information which could be gathered from the literature, we tried to propose a scheme of classification of the observed lesions, aimed to select the best treatment options according to specific situations, to improve results and minimize patients’ risks of further surgeries.
MATERIALS AND METHODS
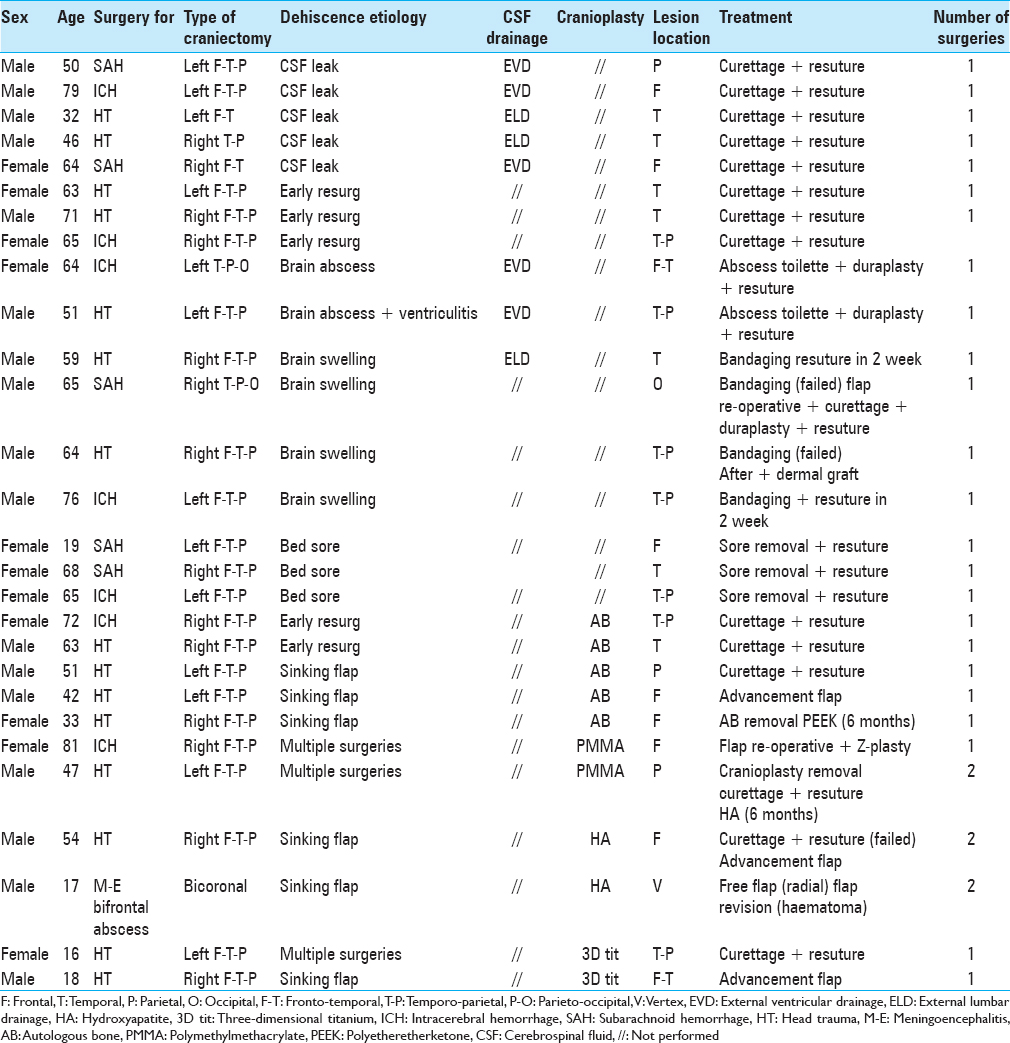
In a 10-year time, from January 2001 to December 2011, 450 DCs were performed at our institution for various etiologies. Two hundred and thirty-five patients underwent decompression because of severe HT, 110 for ICH, 67 for SAH, 14 for hemorrhagic tumors, 11 for massive ischemic brain damage, 9 for meningoencephalitis, 4 for brain abscesses, 5 for bleeding inside arteriovenous malformations, and 5 for brain swelling in malignant brain tumors. Unilateral hemicraniectomy was performed in 362 patients, bifrontal craniectomy in 79, and bilateral hemicraniectomy in 9. Cranioplasty was performed in 344 patients. Autologous bone (AB) was repositioned in 307 cases; artificial implants were used in 37 cases because of AB unavailability (comminuted bone fracture, bone exposure, and bone infection). Polymethylmethacrylate (PMMA), titanium meshes, three-dimensional modeled titanium, and polyetheretherketone (PEEK).
Medium time interval from craniectomy to cranioplasty was 67 days, the shortest time being 27 days, and the longest 158 days. Complications affecting skin flaps were recorded in 38 patients. Standard protocol in all cases included:
Preoperative laboratory examinations: Complete blood count, erythrocyte sedimentation rate, C-reactive protein, and renal and liver function Preoperative computed tomography (CT) scan without and with contrast enhancement, to disclose the presence of subcutaneous, epidural, or intradural (subdural and intraparenchymal) purulent collections, ependymitis, signs of bone, or cranioplasty involvement after reconstruction (osteomyelitis, erosion, and contrast enhancement) 48 h postoperative CT scan.
Antibiotic treatment was distinguished in prophylactic and therapeutic. Prophylaxis (ceftriaxone 1 g 2 h preoperatively, then 6 h after surgery) was used in all cases where no signs of infection could be clearly preoperatively evidenced at the laboratory or instrumental examinations.
Large spectrum therapy (meropenem 1 g 3 times a day, teicoplanin 400 mg as starting dose, then 200 mg two times a day) was started in the immediately postoperative period in cases of inflammatory indexes positivity, clinical evidence of purulent collections, autologous/artificial cranioplasty involvement at neuroradiological examinations, to allow intraoperative sampling for identification of the causative agent. Treatment modifications were decided on the following positivity. Targeted therapy was preoperatively started in the two only patients affected by multiresistant bacteria (Acinetobacter baumannii, Pseudomonas aeruginosa) and prolonged for at least 2 weeks after surgery. On the basis of their appearance, skin flap lesions were classified into three main types, according to a simplified scheme:
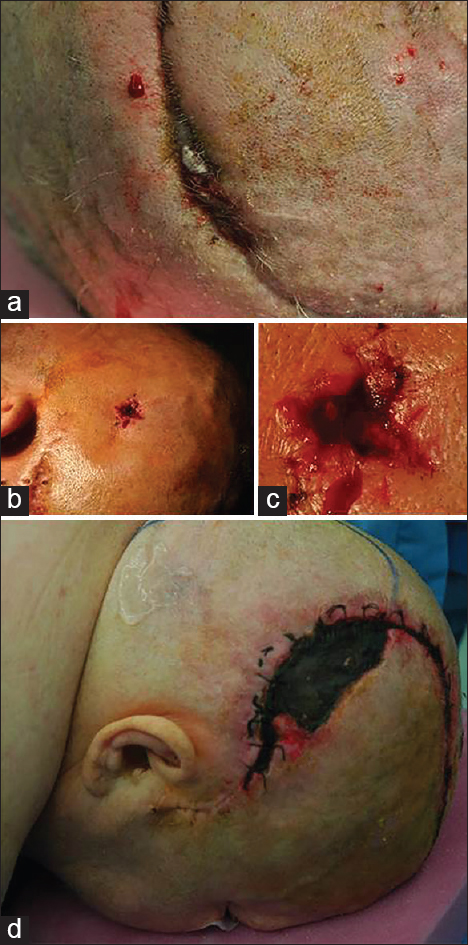
Dehiscence: Defined as a diastase of facing flap borders occurring along the line of suture, with different degrees of exposure of underlying tissues: 28 cases [ Ulcer: Defined as a loss of substance occurring inside the skin flap, usually distant from the line of suture, constantly presenting with underlying tissues exposure: 6 cases [Figure Necrosis: Defined as a large, discolored area of complete loss of skin viability, both on flap contour and on the surrounding skin border, never associated to the exposure of subjacent tissues, always occurring after cranioplasty in unilateral craniectomy, preferentially on the temporoparietal region: 4 cases [Figure
Figure 1
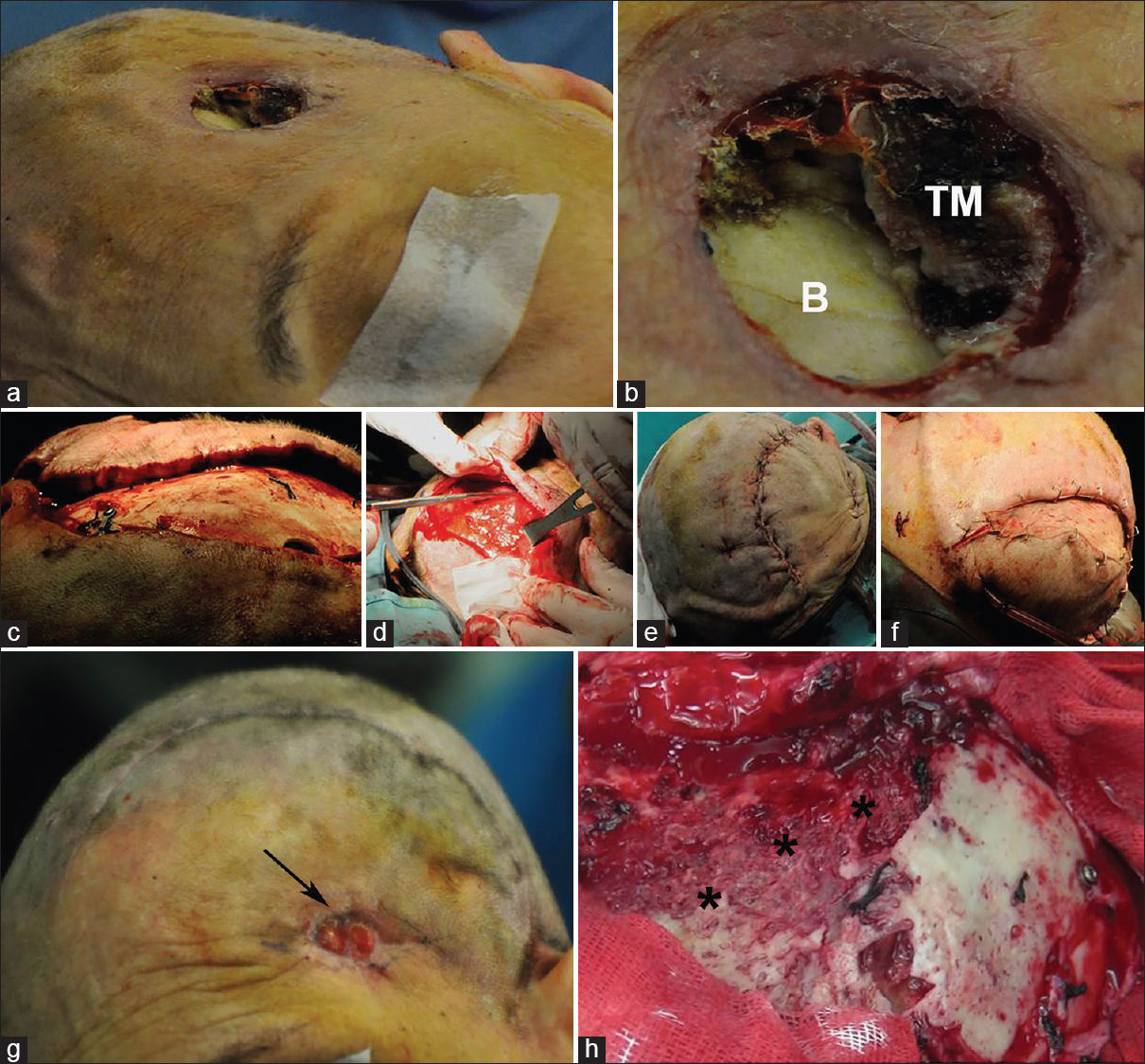
Three different kinds of flap lesions may be observed in patients undergoing decompressive craniectomy or postdecompressive cranioplasty, flap dehiscence, flap ulceration, and flap necrosis. (a) Dehiscence occurring along a left fronto-temporo-parietal decompressive craniectomy. (b) Ulceration occurring in the middle of a left fronto-temporo-parietal flap. (c) In detail, bone is clearly visible under the ulcer. (d) Large necrosis in the temporo-parietal area of a left hemispheric flap
RESULTS
Dehiscence occurred after DC procedures in 17 cases [
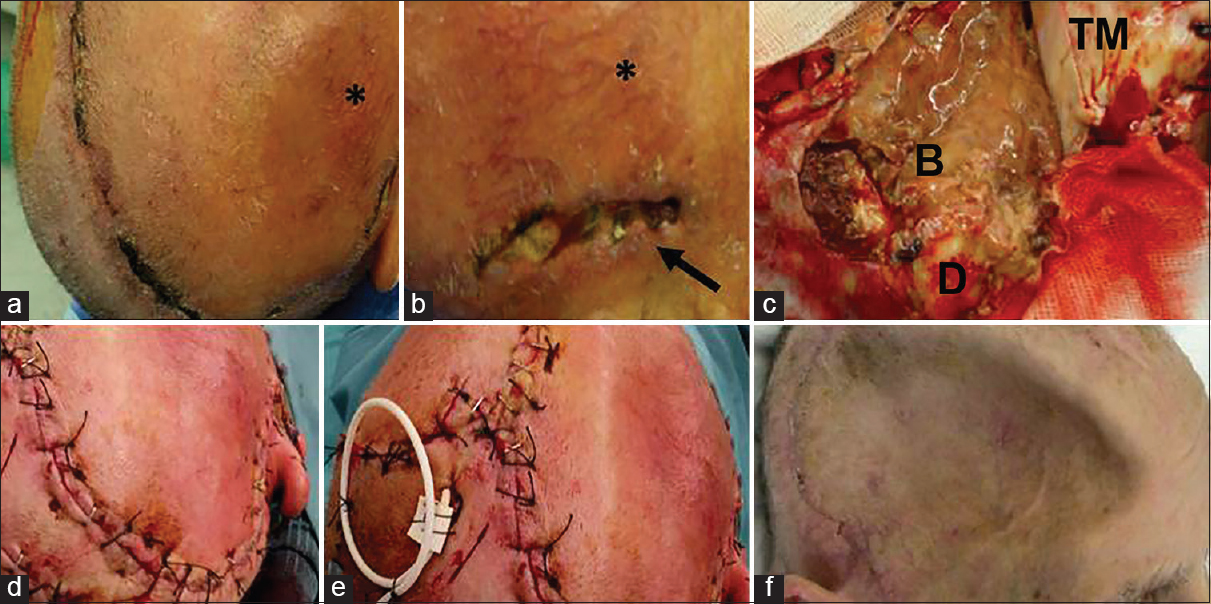
Figure 2
(a and b) 54-year-old male previously treated by the right hemispheric decompressive craniectomy for severe head trauma. Twenty-one days after surgery the flap appeared swollen, showing a reticular pattern of small vessels surrounding the area of dehiscence (black asterisk). A 3 cm long, 1 cm large dehiscence was observed along the temporal line (black arrow). (c) At flap re-opening, after lifting the temporalis muscle (TM), a purulent collection involving the brain was immediately evident (B), with partial resorption of the overlying dural membrane (D). (d) Flap re-suturing was then easily obtained. (e) Intraoperative positioning of a contralateral external ventricular shunt was needed to reduce flap tension allowing uncomplicated re-suturing. (f) One month after revision a sinking flap syndrome developed. Nonetheless, full healing of the skin flap was evident
Dehiscence was also observed after AB cranioplasty (five patients), PMMA (two patients), hydroxyapatite (two patients), and preformed titanium (two patients) [
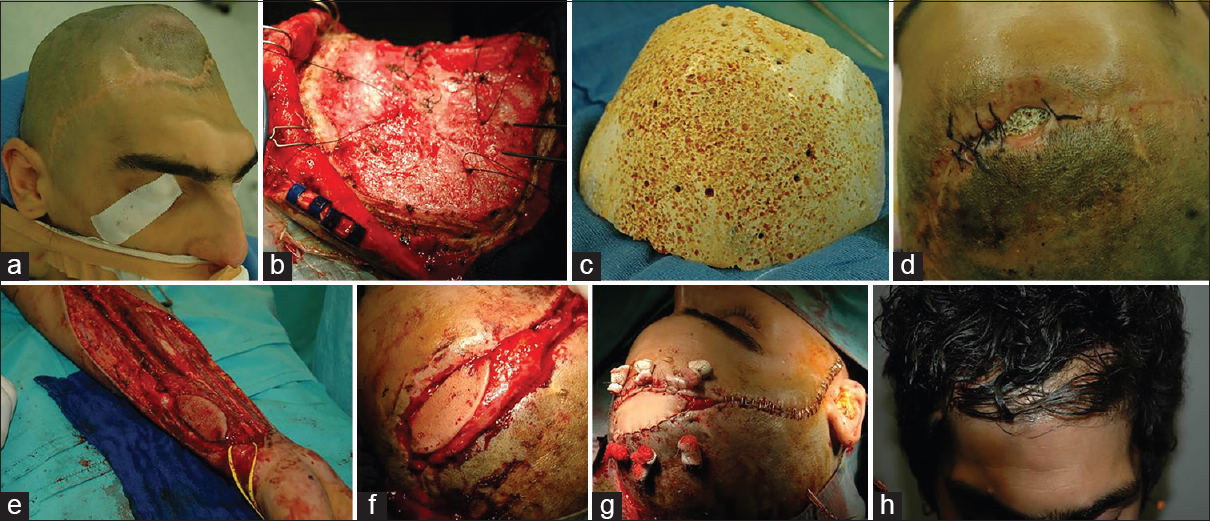
Figure 3
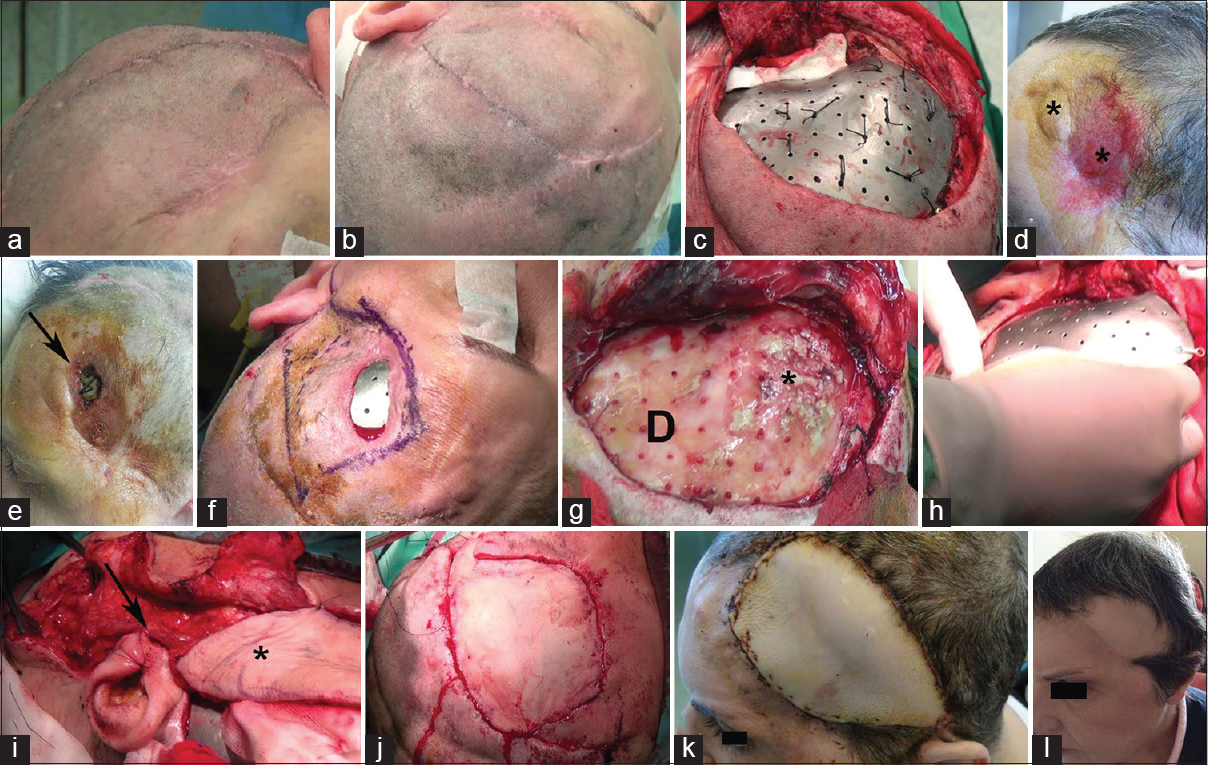
(a) A 21-year-old male undergoing hydroxyapatite cranioplasty after bifrontal decompressive craniectomy for meningoencephalitis. (b and c) Intraoperative images of the flap and the implant. (d) Three weeks after cranioplasty the patient came back to our attention because of a large dehiscence occurring in the middle of the bifrontal flap. (e) A free radial flap from the right forearm was prepared. (f) After performing the anastomosis with the tireo-linguo-facial trunk, the radial flap was positioned over the defect. (g) Final vision after completing flap closure. (h) Four months after surgery full flap healing was observed
Figure 4
(a) Ulceration occurring over the left pterional region 3 months after fronto-temporo-parietal cranioplasty for severe head trauma. (b) Particular of the area of ulceration with fully exposed underlying bone and incomplete necrosis of the temporalis muscle (B: Bone, TM: Temporalis muscle). (c) After fronto-temporo-parietal flap re-opening, bone appeared well-preserved. (d) Preparation of a parieto-occipital advancement flap. (e) Full defect coverage after removal of damaged skin tissue. (f) Detail of the dermal graft needed to close the parietal defect remaining after flap advancement. (g) Three months after flap advancement, relapse of pterional ulcer (black arrow). (h) At flap re-opening evidence of diffuse bone erosion (asterisks), leading to bone removal
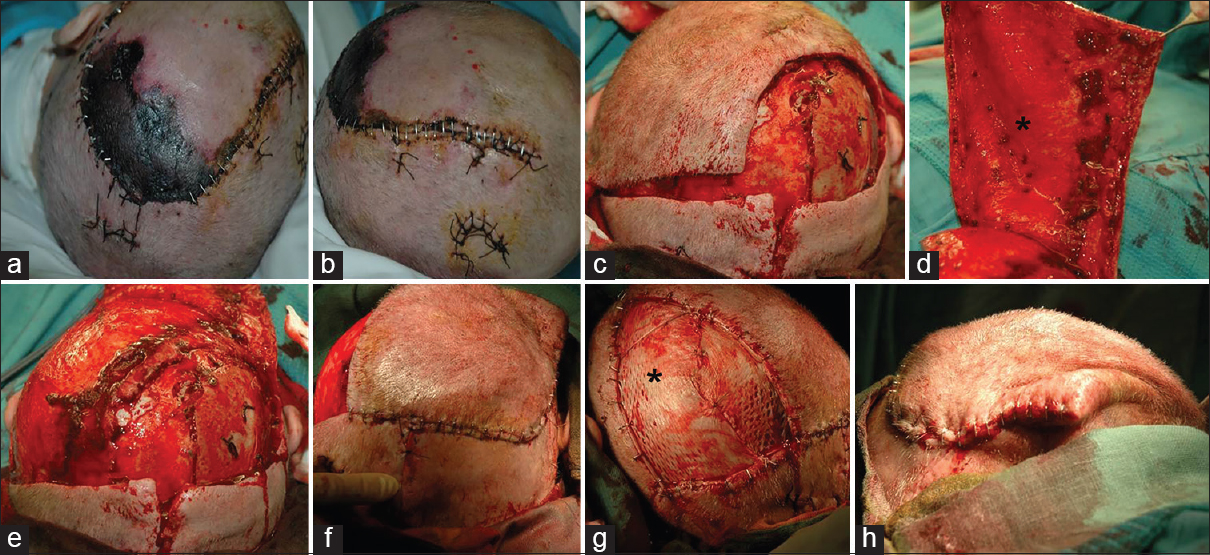
Figure 5
(a and b) Evidence of complete flap healing pretitanium cranioplasty. (c) Final aspect after titanium implant positioning. (d) Flap appearance 3 months after titanium cranioplasty. Even though still intact, a large area of the frontal skin appears thinner and introflexed (asterisks). (e) Two weeks after cranioplasty, the titanium implant got exposed (black arrow). (f) Immediately preoperative planning of skin area needing removal. (g) After cranioplasty removal, the dural layer (D) underwent an accurate curettage, especially over the frontal region, where some flogistic material (asterisk) was observed. No positivity for bacterial or fungine infection resulted from microbiological studies in the following postoperative course. (h) Cranioplasty repositioning after intraoperative sterilization. (i) After vessels anastomosis had been completed, the radial flap (asterisk) was ready to be sutured (black arrow: Site of arterial anastomosis). (j) Radial flap fully covered the defect. All of the damaged skin had been removed. (k and l) Postoperative images 3 weeks and 1 year after surgery, showing full healing
Figure 6
(a and b) A large area of necrosis developed along the temporo-parietal border of a left fronto-temporo-parietal flap 2 days after autologous cranioplasty. (c) A large area of skin flap including the necrotic tissue was removed. (d) Elevation of a parieto-occipital flap (asterisk) to cover the defect. (e) Scalp area exposed after flap elevation. (f) Flap rotation and suture over the previous area of necrosis. (g) Dermal autograft from thigh (asterisk). (h) After flap closure, a small amount of exceeding skin is observed above the ear
DISCUSSION
DC effectiveness is still controverse. Though its positive impact on malignant intracranial hypertension unresponsive to maximal medical treatment is well established, there seems not be an equivalent correlation between patient’s survival rate and good outcomes. To further increase confusion, larger series in literature include patients treated by different techniques, bifrontal craniectomy, hemicraniectomy, and bilateral hemicraniectomy. Bifrontal DC is indicated in patients affected by diffuse traumatic brain injury or in cases where bifrontal contusions are causing mass effect and need prompt surgical evacuation. Hemicraniectomy (fronto-temporo-parietal or fronto-temporo-parieto-occipital) is commonly used in patients harboring unilateral mass lesions (ICH, acute subdural hematoma, and contusions) causing contralateral midline displacement or intracerebral herniations. The use of bilateral DC is infrequent, only occasionally reported and reserved to patients presenting lesions with mass effect evolving at different stages.
Complications occurring after DC and cranioplasty have been described quite recently in a small number of papers.[
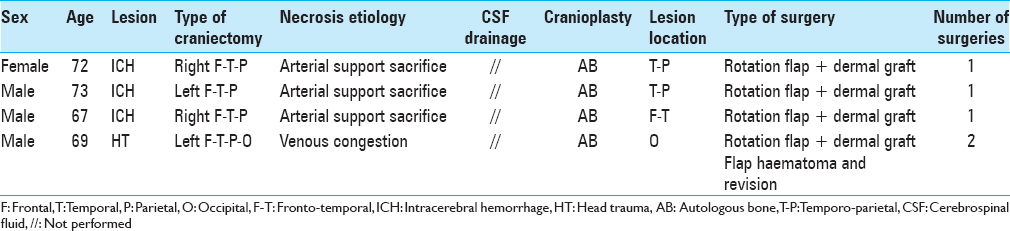
As summarized above, we tried to highlight the etiology of flap lesions case by case. For what is concerning to dehiscences, we observed two leading causes of flap failure after decompression: CSF circulation disturbances and malignant postdecompressive brain swelling. On the counterpart, dehiscences followed cranioplasty essentially because of poor preoperative flap conditions (sinking and multiple surgeries). Ulcers always came associated with an underlying infection of AB or cranioplasty and were never observed in craniectomized patients. In our series, necrosis was ascribed to inadvertent sacrifice of the residual arterial supply after flap reopening in three cases, to venous congestion in 1.
Even if in our experience we were able to find a direct correlation between flap incision and wound complications only in necrosis, some basic principles should be always kept in mind when performing DC. Flap shape needs to be tailored to patient’s anatomy, especially in cases where vascularization may be already compromised (previous surgery on the same side of decompression, scars, irregular scalp lesions as in trauma). ICP monitors must be strategically placed. If put on the same side which could need decompression, it should ideally be placed along the theoretical flap course (frontal region, 2–3 cm lateral to the midline), rather than inside it. In cases where decompression might be required but it is not certain, it is definitely better to raise a larger flap, to avoid last minute transverse incisions, which almost inevitably lead to healing difficulties and increase the risk of breakdown at re-opening. In hemicraniectomy, flap does not need to go beyond the midline, but if a very low frontal access is required (e.g., to access fronto-basal contusions), it is better to shape a curve following the contralateral hairline than cutting a straight line going through the forehead that would presumably compromise the support from supratrochlear and supraorbital arteries. Even if time spending, isolation, and preservation of the superficial temporal artery and the surrounding veins should always be sought, It requires just a few more minutes but reduces significantly the risks of compromise of flap circulation and it is especially valuable in bifrontal DC. To this aim, when starting incision at tragus, there is no need to overrun the zygomatic arch and monopolar coagulation is to be avoided. In patients undergoing hemicraniectomy, the temporal incision should be done 2 cm above the ear, then follow a curve line along the temporal contour at the same level. Because of the poor local vascularization, going below and behind the ear limits the possibility of mobilizing the skin and increases the risks of flap failure. The same exposure of the temporal bone can be obtained with a higher incision and then by retracting the skin by hooks or stitches. The recommended posterior extension of unilateral decompressive flaps is 2–3 cm behind the ear, but to expose enough bone it should be not <5. However, care is not needed to compromise the vascular support from the occipital artery and the posterior midline should not be reached by the incision unless the lesion to treat resides in that area.
Finally, in bifrontal flaps, the coronal portion of incision should preferably follow the coronaric suture or go 2–3 cm behind it.
Coming back to the literature, we would say complications affecting skin flaps after decompression or cranioplasty seem to occupy no space in major authors’ experience, especially in larger series, where we expected the most to find some information. In the work of Honeybul and Ho, no cutaneous problems affecting decompressive flaps were mentioned.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ban SP, Son YJ, Yang HJ, Chung YS, Lee SH, Han DH. Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc. 2010. 48: 244-50
2. Bobinski L, Koskinen LO, Lindvall P. Complications following cranioplasty using autologous bone or polymethylmethacrylate – Retrospective experience from a single center. Clin Neurol Neurosurg. 2013. 115: 1788-91
3. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
4. Honeybul S, Ho KM. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011. 28: 929-35
5. Santana-Cabrera L, Pérez-Acosta G, Rodríguez-Escot C, Lorenzo-Torrent R, Sánchez-Palacios M. Complications of post-injury decompressive craniectomy. Int J Crit Illn Inj Sci. 2012. 2: 186-8
6. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V. Cranioplasty after decompressive craniectomy: The effect of timing on postoperative complications. J Neurotrauma. 2012. 29: 1090-5
7. Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG. Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011. 2: 123-
8. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62