- Department of Neurosurgery, Santa Casa de Misericordia Hospital, Belo Horizonte, Minas Gerais, Brazil
Correspondence Address:
Warley Carvalho da Silva Martins
Department of Neurosurgery, Santa Casa de Misericordia Hospital, Belo Horizonte, Minas Gerais, Brazil
DOI:10.4103/2152-7806.158518
Copyright: © 2015 da Silva Martins WC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Silva Martins WC d, de Albuquerque LA F, de Albuquerque LA F, Dellaretti M, de Sousa AA. Surgical treatment of the intracranial pial arteriovenous fistula. Surg Neurol Int 10-Jun-2015;6:102
How to cite this URL: Silva Martins WC d, de Albuquerque LA F, de Albuquerque LA F, Dellaretti M, de Sousa AA. Surgical treatment of the intracranial pial arteriovenous fistula. Surg Neurol Int 10-Jun-2015;6:102. Available from: http://surgicalneurologyint.com/surgicalint_articles/surgical-treatment-intracranial-pial-arteriovenous-fistula/
Abstract
Background:Pial arteriovenous fistula (PAVF) is a rare vascular condition comprising of one or more arterial vessels that are in direct communication with the draining veins. The condition is also characterized by the absence of a nidus. Due to high blood flows, varicose systems adjacent to the fistula appear. The key characteristic of the arteriovenous direct transition is that it offers a treatment option in which interruption of the blood flow can occur without removing the entire lesion. This study presents two cases of PAVF.
Case Description:The first case is of a 59-year-old male with lesions in the frontal region, fed by a branch of the right anterior cerebral artery and drained by the frontal basal vein to the sphenoparietal sinus. The second case is of a 3-year-old child with a lesion in the right anterior frontal lobe, fed by a branch of the right middle cerebral artery, which drains into the Trolard vein and was associated with large a venous varix.
Conclusion:PAVF is a disease characterized by its rarity, and knowledge of PAVF's clinical presentation is of vital importance in early diagnosis. The treatment of the condition consists of an occlusion of the supply vessel, which can be done by endovascular, microsurgical, or both procedures. Both the cases were successfully treated by microsurgical procedure.
Keywords: Arteriovenous malformation, intracranial, pial arteriovenous fistula
INTRODUCTION
Pial arteriovenous fistula (PAVF) is a rare intracranial vascular lesion characterized by having one or more arterial pedicles in direct connection with a single draining vein.[
Initially, the PAVF was considered a variation of arteriovenous malformation (AVM), however, it has a very different clinical appearance as well as differing therapeutic options.[
CASE REPORTS
Case 1
A 59-year-old male reported that he had suffered a complex partial seizure 19 days before admission. The neurological examination was normal.
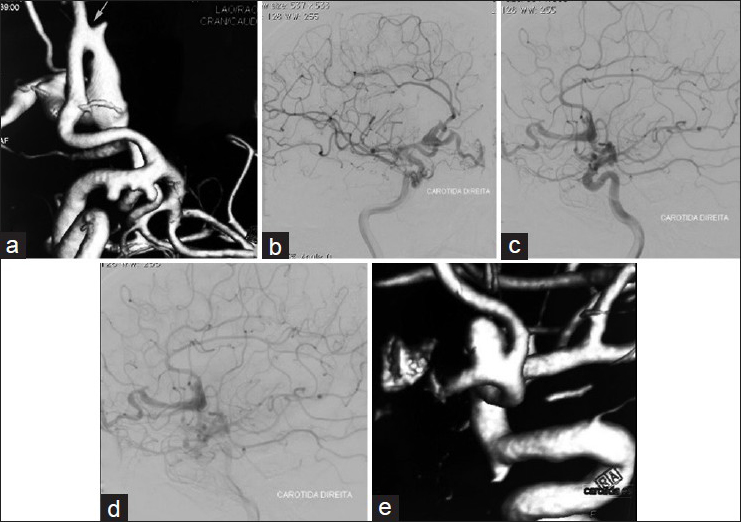
Computer tomography (CT) showed a lesion in the frontal region that suggested an AVM. The cerebral digital subtraction angiography showed a PAVF fed by a dilated branch of the right anterior cerebral artery, draining from the frontal basal vein to the sphenoparietal sinus and dilation of the frontal basal vein [
Figure 1
Preoperative angiography of a PAVF with associated aneurysms. (a) 3D angiography showing a PAVF fed by a branch of the anterior cerebral artery with draining from the frontal basal vein (arrow). Aneurysms in the internal carotid artery at the origin of the posterior communicating artery and anterior choroidal arteries and a pericallosal aneurysm. (b) Oblique view of the angiography showing a PAVF. (c) Side view of the angiography in arterial phase showing a PAVF fed by the anterior cerebral artery. (d) Side view of the angiography in venous phase showing dilated frontal basal vein due to a PAVF. (e) 3D angiography showing aneurysm of the anterior communicating artery
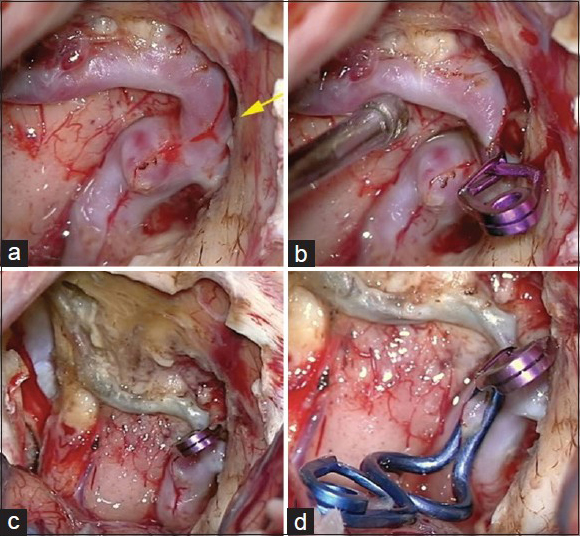
The patient was submitted to a frontal basal craniotomy on the left side, contralateral to the draining vein. The fistula and aneurismatic dilation were occluded with clips [
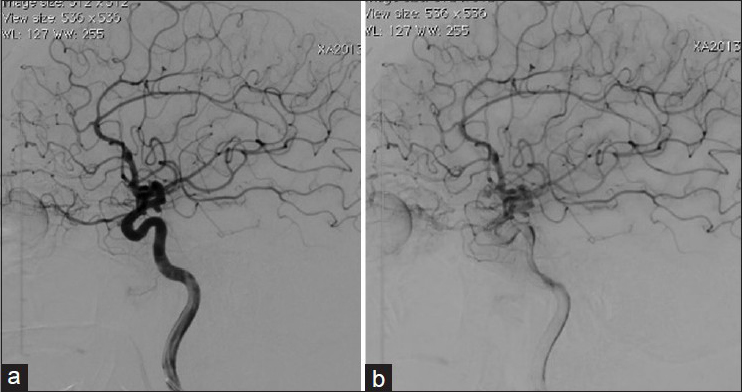
An angiographic exam carried out 6 months after the patient's discharge revealed the persistence of three aneurysms, and a spontaneous regression of the aneurysm on the pericalosa artery. The patient was submitted to a surgical treatment of the aneurysm of the anterior communicating artery and two baby aneurysms on the right internal carotid artery. A postoperative control angiography revealed complete exclusion of these aneurysms.
Case 2
A 3-year-old child, manifesting a progressive loss of strength on the left side of the body over a 6-month period was hospitalized. Neurological examination revealed mild left spastic hemiparesis.
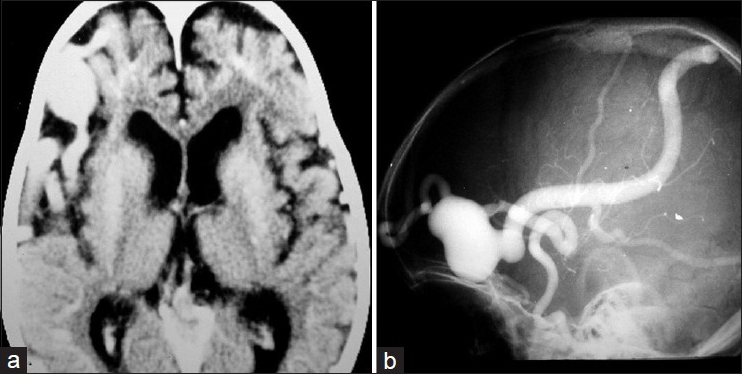
A CT brain scan indicated a frontal-temporal cortical hypotrophy on the right side and a dilated vascular image in the right anterior frontal region suggesting a cerebral AVM [
Figure 4
(a) Brain CT showing a right frontal temporal cortical hipotrophy and extensive vascular dilation in the right anterior frontal region. (b) Cerebral angiography showing a PAVF in the anterior frontal region fed by a branch of the right middle cerebral artery and drained by the swollen Trolard vein with large venous aneurysm
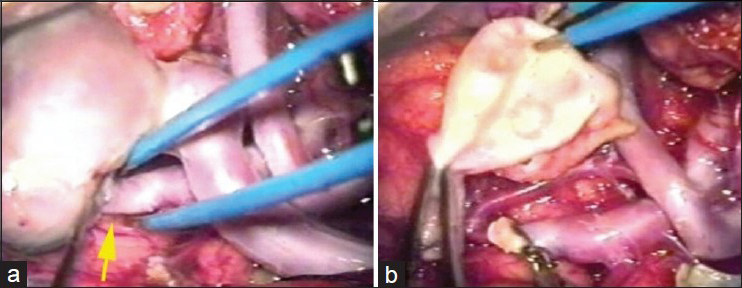
The patient was submitted to a frontal temporal sphenoidal craniotomy on the right side and microsurgical dissection of the Sylvian fissure. The variceal dilatation also caused significant mass effect due to very hard and thick wall. Thus, the PAVF was treated by occlusion of the feeding artery. Resection of variceal dilatation was also performed due to mass effect [
DISCUSSION
Many types of cerebrovascular lesions occur in the central nervous system, each one receiving a different name according to its morphological features. If there are abnormal vessels between the two systems (arterial and venous), they are called nidus and are considered an AVM. If there is no nidus the condition is referred to as arteriovenous fistula (AVF), which can be pial or dural. If the blood vessels are not related to the duramater and come from the cortical and/or pial regions, they are called PAVF.[
The pathophysiology of PAVF includes congenital, traumatic, and iatrogenic etiologies.[
The natural history is unfavorable, especially in children aged less 2 years, and patients with multiple feeding vessels. The mortality rate can reach 63% in cases with conservative treatment.[
Madsen et al.[
The treatment of PAVF consists of an occlusion of the supply vessel. The endovascular procedure has been considered less invasive and is adopted as the treatment of choice by many authors.[
Despite the vast majority of patients with PAFV were treated with endovascular approach, the choice of the treatment should be multidisciplinary and based on the experience of the medical team. It is important to evaluate the location and degree of lesion complexity.[
CONCLUSIONS
PAVF is a very rare disease. This clinical presentation is of vital importance in early diagnosis. The treatment of this condition consists of an occlusion of the supply vessel and the possible approaches employed are microsurgical, endovascular, or combined treatment. Both the cases were successfully treated by microsurgical procedure.
References
1. Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Dowd CF, Barnwell SL. Transarterial occlusion of solitary intracerebral arteriovenous-fistulas. Am J Neuroradiol. 1989. 10: 747-52
2. Han L, Wang JW, Chen JC, Lei T. Pial arteriovenous fistula with giant venous varix and dilation of vein of Galen, treated with surgical clipping. Acta Neurochir. 2013. 155: 1571-2
3. Hoh BL, Putman CM, Budzik RF, Ogilvy CS. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery. 2001. 49: 1351-63
4. Jabbour P, Tjoumakaris S, Chalouhi N, Randazzo C, Gonzalez LF, Dumont A. Endovascular treatment of cerebral dural and pial arteriovenous fistulas. Neuroimaging Clin N Am. 2013. 23: 625-36
5. Lee JY, Son YJ, Kim JE. Intracranial pial arteriovenous fistulas. J Korean Neurosurg Soc. 2008. 44: 101-4
6. Madsen PJ, Lang SS, Pisapia JM, Storm PB, Hurst RW, Heuer GG. An institutional series and literature review of pial arteriovenous fistulas in the pediatric population: Clinical article. J Neurosurg Pediatr. 2013. 12: 7-
7. Moscote-Salazar LR, Alvis-Miranda HR. A proposal for a new pial arteriovenous fistulas grading scale for neuroendovascular procedures and literature review. Rom Neurosurg. 2013. 20: 9-
8. Nakiri GS, Abud TG, Oliveira RS, Santos AC, Machado HR, Abud DG. Endovascular treatment of intracranial pial arteriovenous fistula. Arq Neuropsiquiatr. 2010. 68: 463-5
9. Nelson K, Nimi Y, Lasjaunias P, Berenstein A. Endovascular embolization of congenital intracranial pial arteriovenous fistulas. Neuroimaging Clin N Am. 1992. 2: 9-
10. Panigrahi S, Mishra SS, Das S, Parida DK. Intracerebral pial arteriovenous fistula with large venous varix: A rare case report. Surg Neurol Int. 2013. 4: 22-
11. Paramasivam S, Toma N, Niimi Y, Berenstein A. Development, clinical presentation and endovascular management of congenital intracranial pial arteriovenous fistulas. J Neurointerv Surg. 2013. 5: 184-90
12. Wang YC, Wong HF, Yeh YS. Intracranial pial arteriovenous fistulas with single-vein drainage-Report of three cases and review of the literature. J Neurosurg. 2004. 100: 201-5
13. Yamashita K, Ohe N, Yoshimura S, Iwama T. Intracranial pial arteriovenous fistula-Case report. Neurol Med Chir. 2007. 47: 550-4
14. Yang J, Kwon OK, Oh CW, Hwang G, Song KS, Lee YJ. Surgical flow disconnection of cerebral pial dual-channel arteriovenous fistula with a large varix: The role of anti-platelet agent or anti-coagulation therapy. Childs Nerv Syst. 2013. 29: 1021-5
15. Yang WH, Lu MS, Cheng YK, Wang TC. Pial arteriovenous fistula: A review of literature. Br J Neurosurg. 2011. 25: 580-5
16. Zhang ZY, Yang K, Wang CH, Zhang CW, Xie XD, Tang JJ. Congenital pial arteriovenous fistula in the temporal region draining into cavernous sinus: A case report. Korean J Radiol. 2013. 14: 497-500