- Department of Neurosurgery, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
- Department of Internal Medicine, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
- Department of Neurosurgery, Razi Hospital, Zahedan University of Medical Sciences, Saravan, Iran
Correspondence Address:
Abbas Amirjamshidi
Department of Neurosurgery, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
DOI:10.4103/2152-7806.166194
Copyright: © 2015 Saeedinia S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Saeedinia S, Nouri M, Azarhomayoun A, Hanif H, Mortazavi A, Bahramian P, Yarandi KK, Amirjamshidi A. The incidence and risk factors for surgical site infection after clean spinal operations: A prospective cohort study and review of the literature. Surg Neurol Int 29-Sep-2015;6:154
How to cite this URL: Saeedinia S, Nouri M, Azarhomayoun A, Hanif H, Mortazavi A, Bahramian P, Yarandi KK, Amirjamshidi A. The incidence and risk factors for surgical site infection after clean spinal operations: A prospective cohort study and review of the literature. Surg Neurol Int 29-Sep-2015;6:154. Available from: http://surgicalneurologyint.com/surgicalint_articles/the-incidence-and-risk-factors-for-surgical-site-infection-after/
Abstract
Background:Postoperative infection is one of the most common complications after spine surgeries. In our study, surgical site infection (SSI) is described as; superficial (i.e., skin and subcutaneous tissues) and deep (i.e., fascia and muscles) infections occurring in the short term (i.e., 1-month) after spine surgeries (Centers for Disease Control and Prevention definition 81.00–81.08). To detect the risk factors for the occurrence of such a complication, studies require a large number of patients, a high quality of data and adequate analysis. In this study, we prospectively enrolled 987 patients undergoing spinal surgery over a 3 years period.
Methods:From November 2010 to November 2013, 987 patients had a variety of spinal operations that included; disc herniation, spinal stenosis, spondylolisthesis, fracture-dislocations, spine and spinal cord tumors, and syringomyelia. Patients under the age of 10, those with a recent history of infection and antibiotherapy, and patients with immunodeficiency disorders were excluded.
Results:Of the 987 spine procedures performed, 27 (2.73%) developed postoperative infections. Multi-variant data analysis indicated that multiple factors correlated with an increased risk of SSI in descending order; trauma, a past history of diabetes, smoking, being confined to bed, in the perioperative period, mean blood sugar levels above 120 mg/dl, longer lengths of incisions, and longer hospital stay.
Conclusion:Considering the preventable nature of most of the factors contributing to SSI, it should be possible to reduce these complications.
Keywords: Diabetes, discectomy, spine fusion, spine trauma, surgical site infection
INTRODUCTION
In spine surgery, following pneumonia and urinary tract infections, surgical site infection (SSI)[
METHODS
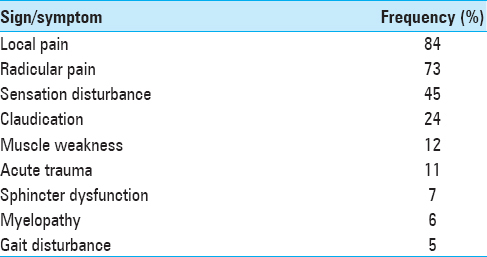
Population from November 2010 to November 2013, 1042 patients underwent spine surgery at one institution. Patients were excluded due to: Death attributed to noninfectious causes (4 patients), those who received previous antibiotic-therapy/had history of infection/those requiring prophylactic antibiotics (e.g., history of endocarditis) (19 patients), age under 10, immunodeficiency disorders, or lost to follow-up (19 patients). The 987 patients with variable symptoms [
Antibiotic therapy prophylaxis
Cephazolin (1 g for patients lighter than 70 kg and 2 g for those heavier) was injected intravenously half an hour prior to the skin incision.[
Surgical measures to reduce surgical site infection
The various prophylactic measures were undertaken to reduce the risk of SSI. These included; irrigation at the end of surgery with normal saline/gentamicin, utilization of a closed drainage system for 24 h, and routine discharge on the second postoperative day.
Postoperative follow-up
Postoperative surveillance for infection included; suture removal 2 weeks postoperatively and follow-up for the first postoperative month. Those with suspected SSI were hospitalized for further evaluation; both superficial and deep SSIs were considered “unfavorable outcomes” [Tables
Statistical analysis
Data were imported to SPSS software version 20 (South Melbourne, Victoria: Cengage Learning Australia, 2012, SPSS Inc.) for statistical analysis. Chi-square (χ2) test, analysis of variance, multi-variant and logistic regression analyses were utilized to compare the data when indicated. Considering the heterogeneity of the pathologies and the original diseases, measurement of the odd's ratio of each variable could not address any idea. Statistical significance was defined as P < 0.05. Data are presented as mean ± standard error of the mean or in average rates accordingly.
RESULTS
Demographics
Of the 978 patients included in the study 541 (54.8%) were male and 446 (46.2%) were female; ages ranged from 13 to 85 years (mean 46.6). Patients had surgery for a variety of diagnoses [Tables
Multiple surgical parameters
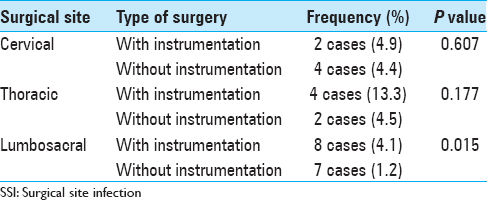
The average levels of discectomy, laminectomy, fusion, the previous surgeries were analyzed. Instrumentation was employed in 264 (26.7%) of patients [
INFECTION PREVALENCE AND ORGANISMS
Twenty-seven (2.7%) patients developed either superficial (25 patients) or deep (2 patients) wound infections. All were treated with intravenous antibiotics (first empirically and then according to the results of culture and antibiogram). None of those with superficial infections developed subsequent deep infections (e.g., spondylodiscitis, osteomyelitis, or meningitis). Culture results included; Staphylococcus aureus in 14 (52%), Acinetobacter in 3 (11%), Streptococcus pneumoniae in 1 (4%) patients, and no organism 9 (33%). Seventeen patients (63%) underwent re-exploration for debridement of the necrotic infected tissues at the surgical site. Only 2 patients (7%) required removal of instrumentation.
Comorbid factors contributing to infection risk: The following comorbid factors correlated with an increased risk of SSI: 72 (7.3%) had DM, 87 (8.8%) hypertension [
Trauma (P < 0.05) A prior history of diabetes (P < 0.05) Smoking in the preoperative period (P < 0.0001) Being bed ridden (P < 0.05) postoperatively Blood glucose levels higher than 120 mg/dl during the period of hospitalization (P < 0.005) The length of incision (P < 0.01) Hospital length of stay (P < 0.005).
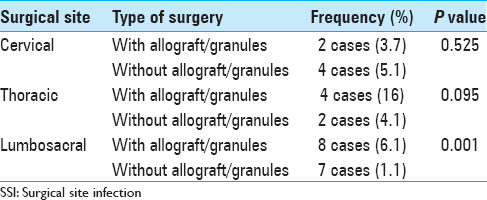
There was no adequate documentation that allograft or hydroxyapatite granules increase the risk of SSI, (P = 0.076).
DISCUSSION
Incidence
The incidence of SSI is reported to be between 1% and 10% in different series.[
Epidemiologic factors
Fang et al. reported age >60 as a predictor of postoperative infections[
Olsen et al. proposed the presence of more than one resident at surgeries as a risk factor.[
Co-morbidities, underlying diseases, and drugs
Higher rate of infections in those younger than 20 in our study, relative immunodeficiency and disturbed defense mechanisms with aging, diabetes, and malignancies in the elderly can be explained by the higher rate of traumatic or tumoral etiologies in each extreme of age [
Logistic regression analysis of the variants in our study, showed that being bed ridden for a long time before and after surgery is an independent imposing factor for SSI after spine surgeries (P < 0.05) [
Regression analysis of our results showed DM as an independent risk factor for postoperative infections (P < 0.005) [
A couple of studies have paid attention to the role of cardio-vascular diseases in the development of postoperative infections.[
Most studies suggest that high BMI promotes postoperative infections[
Analysis of our data shows that smoking is an independent risk factor for SSI (P < 0.0001) [
Anemia is considered as an important risk factor for SSI in head and neck, gynecologic, and colorectal surgeries.[
Surgical strategies and surgery-related factors
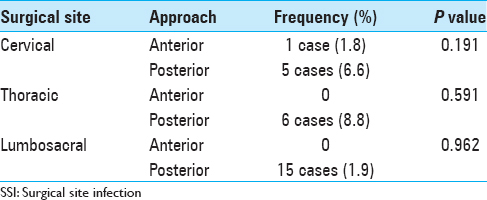
Posterior approaches are associated with higher chance of infection due to long time retraction of the skin and muscles.[
Instrumentation has been considered as a risk factor for development of postoperative infections.[
Leakage of cerebrospinal fluid (CSF) was not an independent factor in our analysis. However, the analysis by Koutsoumbelis et al. considered dural tear as an independent predictive factor SSI.[
The length of skin incision was an independent predictor factor of SSI in our series (P < 0.01) [
Several studies have referred to the role of blood loss and transfusion in higher postoperative infection rate.[
In our series, the increased number of laminectomies or fusions was accompanied by a higher rate of SSI, even though not independently. Similar results were reported previously.[
Miscellaneous factors
Location of the spinal pathology is also believed to affect the rate of infection as the cervical surgeries with low risk[
Longer postoperative immobility and catheterization can decrease wound perfusion (P < 0.005), but it does not seem to be an independent predictive factor for SSI. Immobile patients are those with advanced neurological deficits, CSF leakage and more complex surgeries which all contribute to the increased risk of wound infection.
Limitations
Even though the cases studied in this series is the largest reported in the literature, but it contains a heterogeneous group of patients treated consecutively in our department. Well, controlled larger cohorts may be needed for validation of our results especially without the bias of awareness of the surgeons to undertake anything more than the routine treatment protocol.
CONCLUSION
Most of the elucidated factors (even the rate of accidents in the younger age patients) can be somehow controlled to reduce the risk of infection. Advising the patients to wear correct helmets, lower speed of the motor vehicles, cease smoking before elective surgeries, tight control of blood sugar to <120 mg/dl, weight loosing, and earlier mobilization and rehabilitation after surgery which can reduce the SSI. A long surgery with excessive blood loss should be avoided as much as possible. Undoubtedly, the surgeon's experience and knowledge to make proper decisions in the optimal period of time is of utmost importance in reducing the complication rates including postoperative infections.
References
1. Abdul-Jabbar A, Takemoto S, Weber MH, Hu SS, Mummaneni PV, Deviren V. Surgical site infection in spinal surgery: Description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine (Phila Pa 1976). 2012. 37: 1340-5
2. Last accessed on 2014 Sep 20. Available from: http://www.cdc.gov/ncidod/hip/INFECT/parvo.htm .
3. Last accessed on 2014 Sep 20. Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf .
4. Chikawa T, Sakai T, Bhatia NN, Sairyo K, Utunomiya R, Nakamura M. Retrospective study of deep surgical site infections following spinal surgery and the effectiveness of continuous irrigation. Br J Neurosurg. 2011. 25: 621-4
5. Cizik AM, Lee MJ, Martin BI, Bransford RJ, Bellabarba C, Chapman JR. Using the spine surgical invasiveness index to identify risk of surgical site infection: A multivariate analysis. J Bone Joint Surg Am. 2012. 94: 335-42
6. Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005. 30: 1460-5
7. Fraccalvieri D, Kreisler Moreno E, Flor Lorente B, Torres García A, Muñoz Calero A, Mateo Vallejo F. Predictors of wound infection in elective colorectal surgery. Multicenter observational case-control study. Cir Esp. 2014. 92: 478-84
8. Gruskay J, Smith J, Kepler CK, Radcliff K, Harrop J, Albert T. The seasonality of postoperative infection in spine surgery. J Neurosurg Spine. 2013. 18: 57-62
9. Hayama M, Akahani S, Michiba T, Cho H, Yamamoto M, Mori T. Significant factors for surgical site infection: Analysis of 203 head and neck surgeries. Nihon Jibiinkoka Gakkai Kaiho. 2014. 117: 103-10
10. Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987. 42: 241-9
11. Ishii M, Iwasaki M, Ohwada T, Oda T, Matsuoka T, Tamura Y. Postoperative deep surgical-site infection after instrumented spinal surgery: A multicenter study. Global Spine J. 2013. 3: 95-102
12. Koutsoumbelis S, Hughes AP, Girardi FP, Cammisa FP, Finerty EA, Nguyen JT. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011. 93: 1627-33
13. Lee MJ, Cizik AM, Hamilton D, Chapman JR. Predicting surgical site infection after spine surgery: A validated model using a prospective surgical registry. Spine J. 2014. 14: 2112-7
14. Lonjon G, Dauzac C, Fourniols E, Guigui P, Bonnomet F, Bonnevialle P. Early surgical site infections in adult spinal trauma: A prospective, multicentre study of infection rates and risk factors. Orthop Traumatol Surg Res. 2012. 98: 788-94
15. Mahdi H, Gojayev A, Buechel M, Knight J, SanMarco J, Lockhart D. Surgical site infection in women undergoing surgery for gynecologic cancer. Int J Gynecol Cancer. 2014. 24: 779-86
16. Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003. 98: 149-55
17. Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008. 90: 62-9
18. Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009. 34: 1422-8
19. Pull ter Gunne AF, Mohamed AS, Skolasky RL, van Laarhoven CJ, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976). 2010. 35: 1323-8
20. Quintiliani L, Pescini A, Di Girolamo M, Iudicone P, Martini F, Guglielmetti M. Relationship of blood transfusion, post-operative infections and immunoreactivity in patients undergoing surgery for gastrointestinal cancer. Haematologica. 1997. 82: 318-23
21. Satake K, Kanemura T, Matsumoto A, Yamaguchi H, Ishikawa Y. Predisposing factors for surgical site infection of spinal instrumentation surgery for diabetes patients. Eur Spine J. 2013. 22: 1854-8
22. Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010. 19: 1711-9
23. Schwarzkopf R, Chung C, Park JJ, Walsh M, Spivak JM, Steiger D. Effects of perioperative blood product use on surgical site infection following thoracic and lumbar spinal surgery. Spine (Phila Pa 1976). 2010. 35: 340-6
24. Sørensen LT. Wound healing and infection in surgery: The pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: A systematic review. Ann Surg. 2012. 255: 1069-79
25. Thomsen T, Tønnesen H, Møller AM. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg. 2009. 96: 451-61
26. Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009. 34: 1869-72
27. Woods BI, Rosario BL, Chen A, Waters JH, Donaldson W, Kang J. The association between perioperative allogeneic transfusion volume and postoperative infection in patients following lumbar spine surgery. J Bone Joint Surg Am. 2013. 95: 2105-10
28. Xiao B, Tian W, Liu B, Lü YW, Jin PH, Yan K. Impact of short-time treatment of prophylactic antibiotics for surgical site infection in cervical spinal surgery. Zhonghua Yi Xue Za Zhi. 2012. 92: 2764-7