- Department of Neurosurgery, School of Medicine, Keio University, Tokyo, Japan
- Division of Diagnostic Pathology, Keio University Hospital, Tokyo, Japan
Correspondence Address:
Ryosuke Tomio
Division of Diagnostic Pathology, Keio University Hospital, Tokyo, Japan
DOI:10.4103/2152-7806.185008
Copyright: © Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tomio R, Yoshida K, Kohno M, Kamamoto D, Mikami S. The outermost “dura-like membrane” of vestibular schwannoma. Surg Neurol Int 29-Jun-2016;7:71
How to cite this URL: Tomio R, Yoshida K, Kohno M, Kamamoto D, Mikami S. The outermost “dura-like membrane” of vestibular schwannoma. Surg Neurol Int 29-Jun-2016;7:71. Available from: http://surgicalneurologyint.com/surgicalint_articles/outermost-dura%e2%80%91like-membrane-vestibular-schwannoma/
Abstract
Background:The membranous structure of vestibular schwannoma is an important factor in its surgical treatment. Herein, we report intraoperative and microscopic findings relating to an outermost dura-like membrane in cases of vestibular schwannoma and the importance of these findings.
Methods:Intraoperative findings of 16 cases of vestibular schwannoma treated with an initial surgery were studied with an aim to determine if the cases had a dura-like membrane. Then we studied microscopic findings of the dura-like membrane using hematoxylin and eosin, Masson trichrome, and immunohistochemical staining in 2 cases.
Results:The dura-like membrane was observed in 8 out of 16 cases. The average tumor size of the cases that had a dura-like membrane was 30 ± 8.1 mm, and Koos grading 4 was in 7 out of 8 cases, and one was grade 3. In cases without a dura-like membrane, these values were significantly smaller, with an average tumor size of 12.8 ± 5.2 mm, and Koos grading 4 was only in 1 of 8 cases, grade 3 was in 2 cases, and other 5 cases were grade 2. The outermost dura-like membrane enveloped the vestibular schwannoma around the internal acoustic meatus and was continuous with the dura mater. Reactive angiogenesis was observed in the dura mater. Microscopic findings proved its continuity with the dura mater. In one case, the facial nerve was damaged before it was identified during subcapsular dissection. In that case, the dura-like membrane negatively affected our ability to identify the facial nerve.
Conclusions:A dura-like membrane sometimes envelops vestibular schwannoma around the internal acoustic meatus. Recognition of this membranous structure is important for the surgical preservation of facial and acoustic nerves.
Keywords: Acoustic neurinoma, acoustic neuroma, dura mater, operation, surgery, vestibular schwannoma
INTRODUCTION
Preservation of the cochlear nerve and hearing capability remains challenging during surgery for large vestibular schwannoma, however, preservation of the facial nerve function is mandatory in this era. Recognition of the membranous structure and surgical cleavage plane of vestibular schwannoma is important for the preservation of function as well as intraoperative monitoring. The membranous structure of vestibular schwannoma has been reported by many authors to date.[
MATERIALS AND METHODS
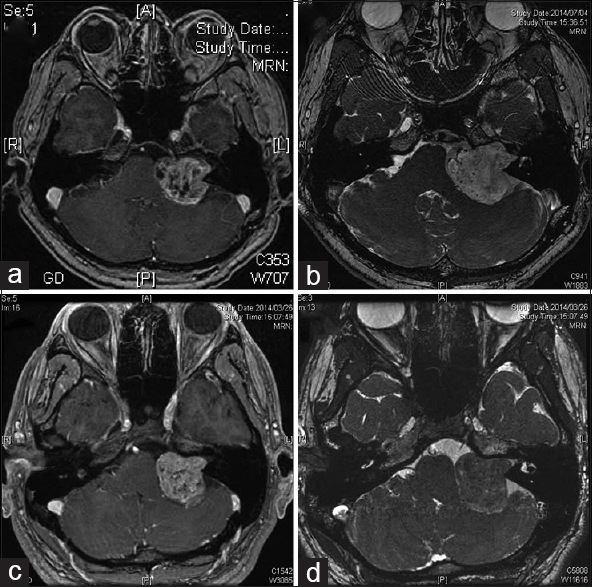
Intraoperative findings of 16 cases of vestibular schwannoma treated with an initial surgery using a lateral suboccipital approach (retrosigmoid approach) between February and March 2015 were prospectively studied with an aim to determine if the cases had a dura-like membrane. Cases of neurofibromatosis type 2 vestibular schwannomas were excluded. The tumor size, Koos grading, and existence of a contact between the dura mater of the petrous bone and the tumor were also studied by magnetic resonance imaging (MRI). All cases were operated upon in the prone-lateral or park-bench position. Facial nerve free-run and evoked electromyogram (EMG), facial nerve motor evoked potential (MEP), and acoustic brain stem responses (ABR) were monitored intraoperatively.
We studied the microscopic findings related to the dura-like membrane in two cases of vestibular schwannoma. In both the cases, the Koos grading of the tumor was 4, and the maximal tumor diameter was 37 mm [
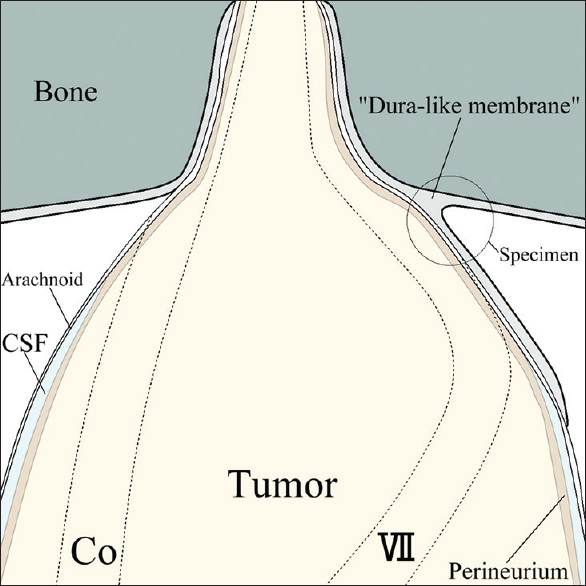
A continuous sheet of the dura-like membrane was taken from the dura mater on the petrous bone just behind the IAM to the tumor surface, and was used as a specimen for microscopic examination.
Microscopic findings of the dura-like membrane were studied on a paraffin-embedded specimen, using classical hematoxylin and eosin (H and E), Masson trichrome (MT), and immunohistochemical staining. S-100, myelin basic protein (MBP), neurofilament (NF), and epithelial membrane antigen (EMA) stains were used for the first case. S-100 and MBP stains were used for the second case. The specimens were sectioned in a plane perpendicular to the dura-like membrane surface to contain the posterior wall side and the tumor side in each section.
This study was conducted in accordance with the ethical standards of the ethics committee of Keio University School of Medicine and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
RESULTS
Characters of cases with the dura-like membrane
The dura-like membrane was observed in 8 of 16 cases. The average tumor size of the cases that had a dura-like membrane was 30 ± 8.1 mm, and Koos grading 4 was in 7 out of 8 cases, another one was grade 3. In cases without a dura-like membrane, these values were significantly smaller, with an average tumor size of 12.8 ± 5.4 mm (t (N = 16) = 5.15, P < 0.01) and Koos grading 4 was only 1 in 8 cases, grade 3 was in 2 cases, and other 5 cases were grade 2. All the tumors with the dura-like membrane were Koos grade 3 or 4 and larger than 24 mm. The dura-like membrane existed in all 6 cases (100%), in which a contact between the dura mater of the petrous bone and the tumor was confirmed in MRI, however, this membrane was observed in only 2 out of 10 cases (20%) that had no such contact.
Intraoperative findings related to the dura-like membrane
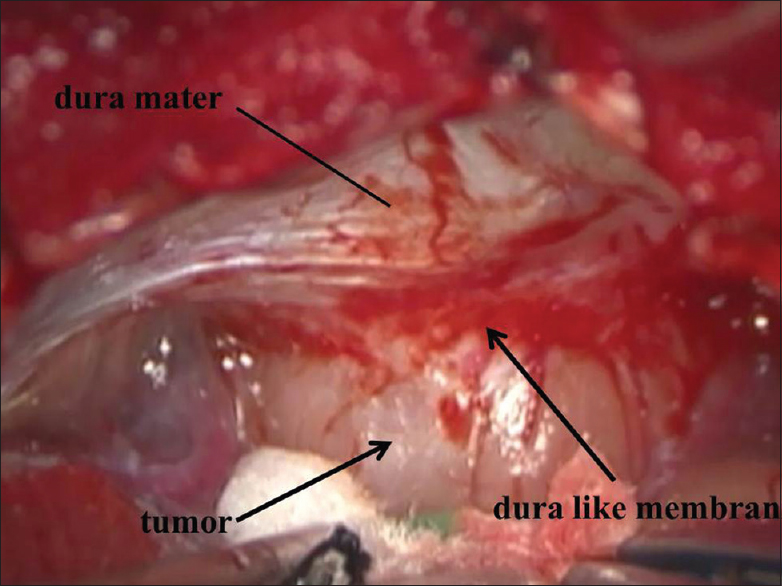
Intraoperative findings showed that the outermost dura-like membrane enveloped the tumor around the IAM, but it was not fully circumferential in most of the cases. The size of the enveloped area varied among cases [Figures
Operation and clinical course
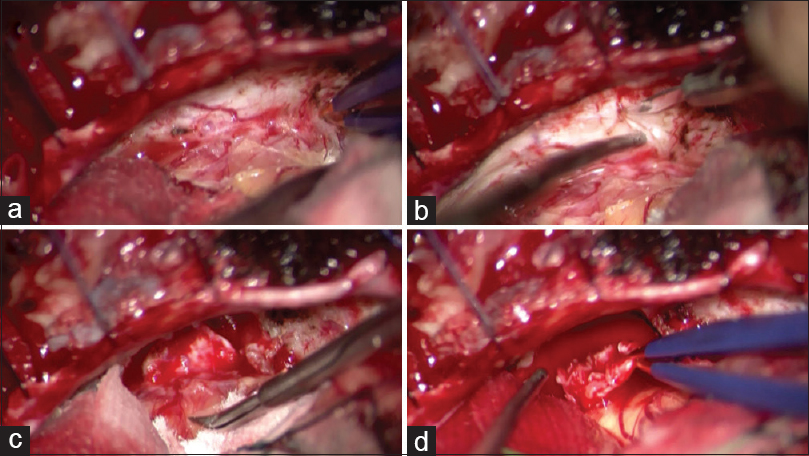
The dura mater of the petrous bone just behind the IAM was peeled off, and then “the posterior wall of the IAM was carefully drilled into. We usually try to confirm the location of the facial nerve by facial EMG monitoring at the IAM and identify a border between the tumor and the facial nerve. A specimen of the dura-like membrane was taken after confirmation that there was no nerve just under the specimen in two cases [
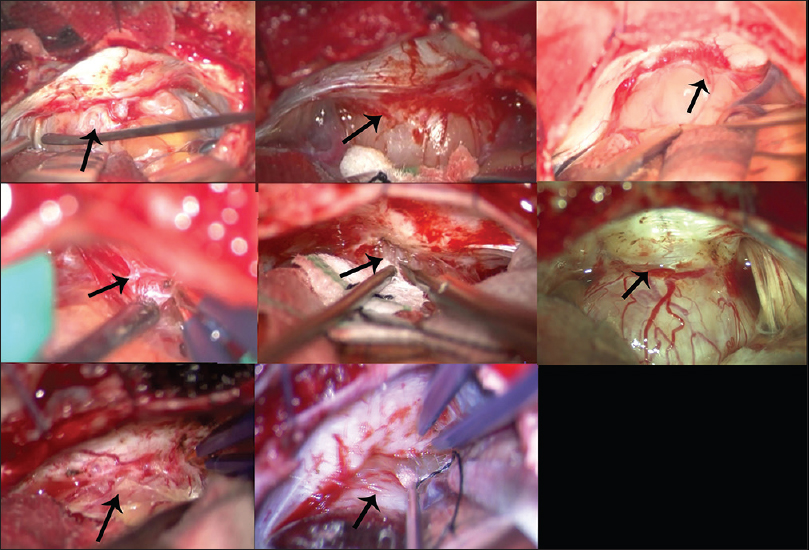
Figure 4
Photographs of operative procedures for harvesting a specimen of the “dura-like membrane” in the first case. (a) The outermost dura-like membrane envelops a part of the tumor around the internal acoustic meatus (IAM) (black arrow). The dura-like membrane appears to be an extension of the dura mater, and this layer seems to trail over the tumor. The membrane contains numerous microscopic vessels, and it appears to provide vascular supply to the tumor. Angiogenesis is also observed on the contiguous dura mater (white arrow). (b-d) A specimen of the dura-like membrane is taken from the dura mater, consisting of a continuous sheet from the surface of the posterior wall of the IAM to the tumor surface (black arrow)
We usually perform a subcapsular dissection of vestibular schwannoma. The outer layer of the tumor forms a “capsule” that is derived from the tumor origin nerve, and this capsule is usually not removed at our institution in order to preserve nerve function.
Facial nerve function was preserved in 75% (6 of 8) of the cases with the dura-like membrane. In one of the cases used for microscopic observation, the facial nerve was severed before it was identified during subcapsular dissection around the IAM. The presence of the dura-like membrane would negatively affect the surgical outcome in this case. The dura-like membrane was fibrous and surrounded the other structures. The facial nerve and the tumor were tightly attached to the dura-like membrane, and hence it was difficult to recognize the facial nerve. We were unable to confirm the facial nerve by EMG stimulation until tumor removal was finished, at which point a distal edge of the severed facial nerve was confirmed. The patient complained of HB grade 6 facial paralysis immediately following the surgery. Another HB grade 3 facial paralysis was observed in a case after the surgery. The facial nerve and its function were completely preserved in the other 6 cases. The capsule of the tumor remained, however, near-total resection was achieved in 6 cases.
Microscopic findings of the dura-like membrane in two cases
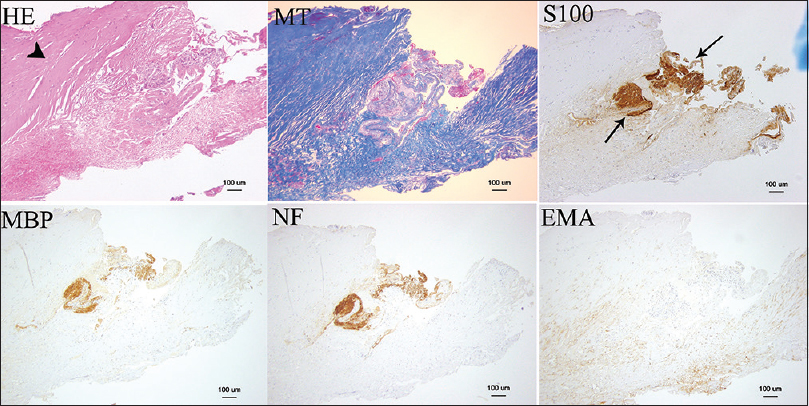
H and E, MT, S-100, MBP, NF, and EMA stains were used for case 1. MT stains myelin (shown in red), NF and MBP stain vestibular nerve fibers, and the schwannoma was stained by S-100. The vestibular schwannoma and vestibular nerve fibers are seen in the middle of the specimen in these stains [
Figure 5
Microscopic findings seen in the first case (×100). Hematoxylin and eosin, Masson trichrome, S-100, myelin basic protein, neurofilament, and epithelial membrane antigen stains are shown. The thick connective tissue layer is the “dura-like membrane” that envelops the vestibular schwannoma (arrow head). S-100 stain showed the tumor (arrow)
Figure 6
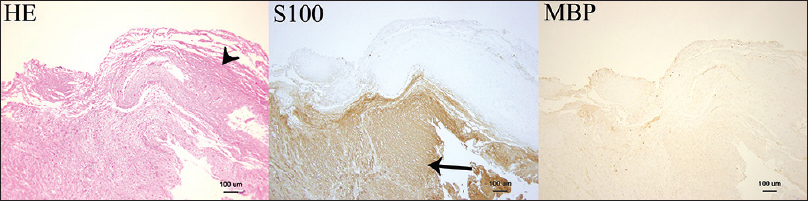
Microscopic findings seen in the second case (×100). Hematoxylin and eosin, S-100, and myelin basic protein (MBP) stains are shown. The tumor is stained with S-100 (arrow), and it is covered by an S-100-negative thick connective tissue layer referred to as the “dura-like membrane” (arrow head). MBP staining is slightly positive at the border between the tumor and the dura-like membrane
DISCUSSION
The dura-like membrane enveloped a section of the vestibular schwannoma around the IAM, and this fibrous layer was tightly attached to the tumor. Vestibular schwannoma usually originates from the inferior or superior vestibular nerve. The facial and cochlear nerves are pressed against the wall of the IAM, and these nerves cling closely to the exterior of the enlarged tumor in the cerebellopontine angle (CPA) cistern part. Thus, the facial and cochlear nerves are usually the most laterally located structures in this surgery. However, the dura-like membrane was located outside of these nerves. This dura-like membrane continued from the dura mater of the petrous bone and enveloped the complex of tumor and nerves from the outside [
Sasaki et al. reported their subcapsular dissection method for vestibular schwannoma surgery.[
The dura-like membrane was apparently continuous with the dura mater, and it appeared to be derived from the contiguous dura mater. Our 8 cases with a dura-like membrane had tumors that were large enough (>24 mm, and Koos grade 3 or 4) to adhere tightly to the dura mater. This contact between the dura mater and the tumor was observed in all cases where the dura-like membrane was seen, and it would thus be one of the required conditions for dura-like membrane origin. A preoperative MRI finding regarding whether there is contact between the dura mater and the tumor was considered a predictive factor for the presence of the dura-like membrane. Reactive angiogenesis within the surrounding dura mater was seen in all cases with a dura-like membrane, and this was apparently induced by the tumor. Koutsimpelas et al. reported that vestibular schwannomas showed expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor at both the mRNA and protein levels.[
Our colleague reported a similar pseudocapsule formation that covered both a trigeminal neurinoma and the trigeminal nerve fibers after gamma knife radiosurgery.[
CONCLUSION
A “dura-like membrane” was sometimes found to envelop a vestibular schwannoma around the IAM. Recognition of this membranous structure is important in vestibular schwannoma surgery, especially for the surgical preservation of the facial and acoustic nerves.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
1. Akiyama T, Ikeda E, Kawase T, Yoshida K. Pseudocapsule formation after gamma knife radiosurgery for trigeminal neurinoma-case report. Neurol Med Chir. 2005. 45: 526-9
2. Kohno M, Sato H, Sora S, Miwa H, Yokoyama M. Is an acoustic neuroma an epiarachnoid or subarachnoid tumor?. Neurosurgery. 2011. 68: 1006-16
3. Koutsimpelas D, Stripf T, Heinrich UR, Mann WJ, Brieger J. Expression of vascular endothelial growth factor and basic fibroblast growth factor in sporadic vestibular schwannomas correlates to growth characteristics. Otol Neurotol. 2007. 28: 1094-9
4. Ohata K, Tsuyuguchi N, Morino M, Takami T, Goto T, Hakuba A. A hypothesis of epiarachnoidal growth of vestibular schwannoma at the cerebello-pontine angle: Surgical importance. J Postgrad Med. 2002. 48: 253-8
5. Sasaki T, Shono T, Hashiguchi K, Yoshida F, Suzuki SO. Histological considerations of the cleavage plane for preservation of facial and cochlear nerve functions in vestibular schwannoma surgery. J Neurosurg. 2009. 110: 648-55
6. Yasargil MG, Smith RD, Gassel JC, Krayenbuhl H.editors. Microsurgical approach to acoustic neurinomas. New York, NY: Springer-Verlag; 1977. 4: 93-129