- Institute of Health Sciences, Hacettepe University, Altindağ 06100, Ankara, Turkey
- Department of Histology and Embryology, Faculty of Medicine, Hacettepe University, Altindağ 06100, Ankara, Turkey
- Department of Molecular Biology and Genetic, Boğazici University, Bebek 34342, Istanbul, Turkey
Correspondence Address:
Gӧkhan Akdemir
Department of Molecular Biology and Genetic, Boğazici University, Bebek 34342, Istanbul, Turkey
DOI:10.4103/2152-7806.173316
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gӧkhan Akdemir, Kaymaz F, Yasemin Gursoy-Özdemir, Akalan N, Akdemir ES. The time course changes in expression of aquaporin 4 and aquaporin 1 following global cerebral ischemic edema in rat. Surg Neurol Int 06-Jan-2016;7:4
How to cite this URL: Gӧkhan Akdemir, Kaymaz F, Yasemin Gursoy-Özdemir, Akalan N, Akdemir ES. The time course changes in expression of aquaporin 4 and aquaporin 1 following global cerebral ischemic edema in rat. Surg Neurol Int 06-Jan-2016;7:4. Available from: http://surgicalneurologyint.com/surgicalint_articles/the-time-course-changes-in-expression-of-aquaporin-4-and-aquaporin-1-following-global-cerebral-ischemic-edema-in-rat/
Abstract
Background:The aim of this global cerebral ischemia study was to study the changes in expression levels of aquaporin 4 (AQP4) and AQP1 over time.
Methods:Sprague-Dawley type male rats were divided into six groups. Sham group and ischemia/reperfusion were performed on five other groups using the four-vessel occlusion model. Reperfusion was done 30 min after the occlusion, and each group was tested at 1, 6, 12, 24, and 48 h for brain wet-dry weight ratio and AQP4 and AQP1 expression levels using immunohistochemistry. To prove ischemia development exists in both hippocampal neurons and epithelia of choroid plexus, hematoxylin, and eosin and neuronal marker (NeuN) immune-staining have been applied to the sham experimental group at 48 h. AQP4 expression levels are also measured with western blotting.
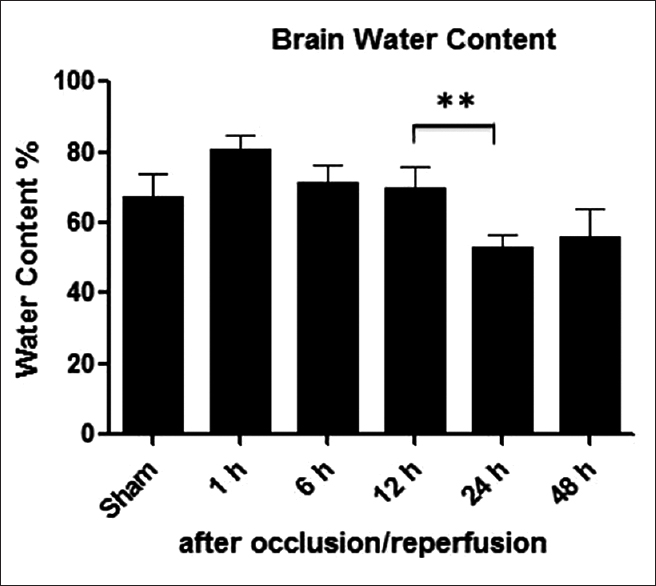
Results:After ischemia/reperfusion it is observed that the decrease in brain water content between 12 and 24 h was statistically significant (P P
Conclusions:Lack of change in AQP4 expression levels is thought as its dual role in formation and elimination of ischemic brain edema. Decrease in AQP1 expression levels in 24 h can be explained with necrosis in choroid plexus after ischemia and the increase in 48 h mark can be related to recovery in choroid plexus.
Keywords: Aquaporin 1, aquaporin 4, brain edema, global cerebral ischemia/reperfusion
INTRODUCTION
Stroke is a major cause of morbidity and mortality in most developed countries.[
Aquaporins (AQPs) are a family of molecular water channel proteins that facilitate water transport across the plasma membranes of cells in response to osmotic gradients.[
AQP4 and AQP1 appear to play critical roles in cytotoxic edema formation and as well as resolution in vasogenic edema. In this study, we explored that the time course changes of AQP4 and AQP1 expressions in a rat model of four-vessel ischemia-reperfusion.
MATERIALS AND METHODS
Animals
Sprague-Dawley type male (weight 180–250 g) rats were used in this study. They were housed under diurnal lightning conditions and fasted overnight but allowed free access to water after the surgical procedure. Animal housing, care, and application of experimental procedures were all carried out in accordance with the institutional guidelines and approved by the Hacettepe University Animal Experiments Local Ethics Committee.
Four-vessel occlusion model
Rats were randomly divided into six groups. One group was the sham group, and ischemia/reperfusion was performed on the other five groups on different hours (1 h, 6 h, 12 h, 24 h, and 48 h) using the four-vessel occlusion model. Rats were anesthetized with intraperitoneal administration of 90 mg/kg ketamine (Ketalar® , Ketamine Hydrochloride Injection, USP, Parke-Davis, Morris Plains, NJ 079950 ABD), and 10 mg/kg xylasine (Rompun®, xylazin hydrochlorid 2%, Bayer, Mefar İlaç San. A.Ş., Istanbul, Turkey).
After confirming deep anesthesia, a midline incision was made with Carl-Zeiss OPMI-1-FC microscope at × 1 to ×1.6 magnifications (Carl Zeiss, GmbH, Germany) in the dorsal neck and the cervical muscles were divided down to the atlanto-occipital junction. To produce experimental global cerebral ischemia, the alar foramina of the atlas were identified, and a bipolar bayonet (Codman Bipolar Johnson and Johnson Co., ABD) was passed in each foramina to electro-coagulate the underlying vertebral artery, and then the muscles and fascia were sutured closed in layers, as a previously reported rat model.[
Brain water content measurement
Brains were removed and divided into an ipsilateral and contralateral hemisphere. The ipsilateral hemisphere tissue was placed on a piece of aluminum foil and weighted immediately (wet weight) and dried in vacuum oven at 70°C for 24 h. The dried brains were reweighted, and brain water content was calculated as (wet weight − dry weight)/(wet weight × 100).
Hematoxylin and eosin staining and immunohistochemistry
Brains were removed control group and at 1 h, 6 h, 12 h, 24 h, and 48 h after four-vessel occlusion. Contralateral hemispheres are waited in fluid nitrogen −196°C for 3 days. Frozen sections of 7 μm thickness were cut in the coronal plane (beginning at the bregma and extending caudally) on a Leica (Jung Frigocut 2800E) cryostat and air dried. After the sections were H and E and examine Leica DM 6000B microscope (Leica Microsystems, Wetzlar, Germany).
The sections were immune-stained at the room temperature for 1 h with rabbit anti AQP4 and AQP1 (1/100 Chemicon AB3594 USA) and mouse anti-NeuN (1:200; Millipore Chemicon USA) followed by the appropriate fluorescent secondary antibody (1:200; Invitrogen-Life Technologies, Carlsbad, CA, USA) or biotinylated secondary antibody (1:500; Vector Laboratories, Burlingame, CA, USA). Tissue sections were examined with a Leica DM 6000B microscope (Leica Microsystems, Wetzlar, Germany).
Immunohistochemistry analysis
Immunoreactivity of the AQP4 and AQP1 was quantified with fluorescence intensity using macnification v1.6.2 analysis software (Orbicule Inc., Leuven, Belgium) with an iMac computer (3.06 GHz Intel Core 2 Duo Apple Co., USA). The level of fluorescence was quantified in the region of interests (ROIs) analysis. AQP4 immunoreactivity was quantified at brain contralateral hemisphere; AQP1 immunoreactivity was quantified in choroid plexus of the third ventricle.
Western blotting of aquaporin 4
Expression of AQP4 was quantified with western blotting. It is performed with the anti-AQP4 antibody (rat, polyclonal, Chemicon, USA). An equal amount of proteins per condition are separated on a 10% Novex Bis-Tris (Invitrogen, USA) gel and electrotransferred onto polyvinylidene fluoride membrane (Invitrogen, USA). Immunoreactive bands were visualized with Kodak Image Station 2000 MM and intensity levels are measured with NIH image program.
Statistical analysis
Immunoreactivity ROI and water content were expressed as mean ± standard error (generally 4–6 mice per each group). A significant difference was defined as P < 0.05. Group comparisons were made with Kruskal–Wallis test. Immunostaining and western blotting results are compared with Wilcoxon test.
RESULTS
Ischemia/reperfusion
We used four-vessel global ischemia model in rat in which permanent occlusion of both vertebral arteries was accomplished by bipolar cautery 24 h before prior to transient bilateral occlusion on the carotid artery using surgical aneurysms clips. Transient bilateral carotid artery occlusion then reduced cerebral blood flow >95%, which was returned to approximately baseline level after reperfusion.
Brain water content
Following four-vessel occlusion/reperfusion ipsilateral hemisphere's water content in the ischemic groups increased gradually until 12 h, and subsequently decreased until 48 h in
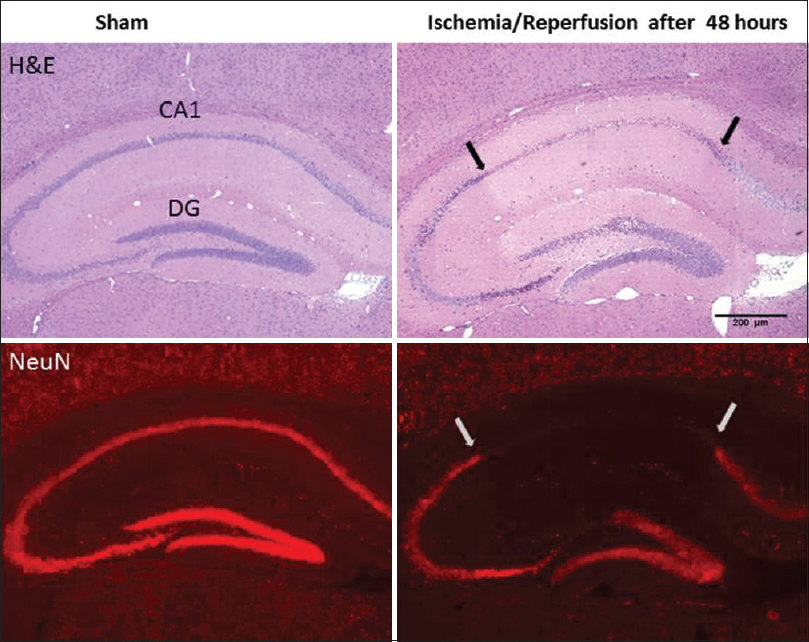
Neuronal damage
Neuronal damage was assessed in brain sections through hippocampus in sham rat and in the ischemic rat at 48 h after four-vessel occlusion.
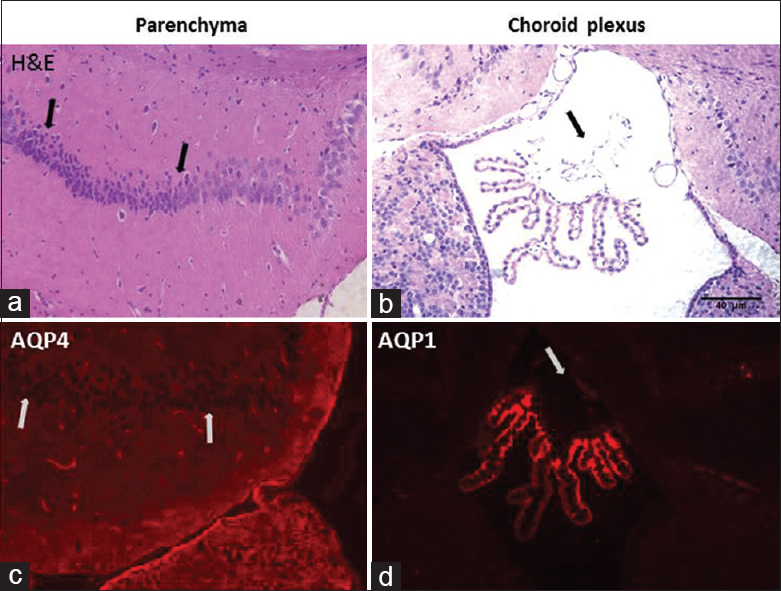
Figure 3
Hematoxylin and eosin staining aquaporins expressions: Histological evaluation with hematoxylin and eosin stained CA1 subregion (a) and choroid plexus of the rat at high magnification (b). CA1 neurons are observed as small, shrunken and dark pyknotic neurons (shown in arrows). Choroid plexus epithelial cells show damage to apical membrane fragmentation and disruption (arrow). Aquaporins expressions are observed by immune-staining of brain parenchyma and choroid plexus in ischemic rats after 48 h. Aquaporin 4 expression is observed in CA1 subregion with stain astrocytes (c). Aquaporin 1 expression is observed in choroid plexus in third ventricle (d)
Choroid plexus damage
Our study considered that not only hippocampal ischemia but also choroid plexus injury. When both vertebral arteries are permanently occluded, and both carotid arteries are temporarily occluded, there is a drastically reduced blood flow to whole brain areas such as hippocampus and choroid plexuses. Four-vessel ischemia-induced anterior and posterior choroidal artery ischemia in rats caused damage of the choroid plexus tissue. We demonstrated that choroid plexus epithelial cells damaged in
Aquaporin 4 and aquaporin 1 expression
We next evaluated the expression of AQP4 and AQP1 after cerebral ischemia/reperfusion injury. The immunostaining results in
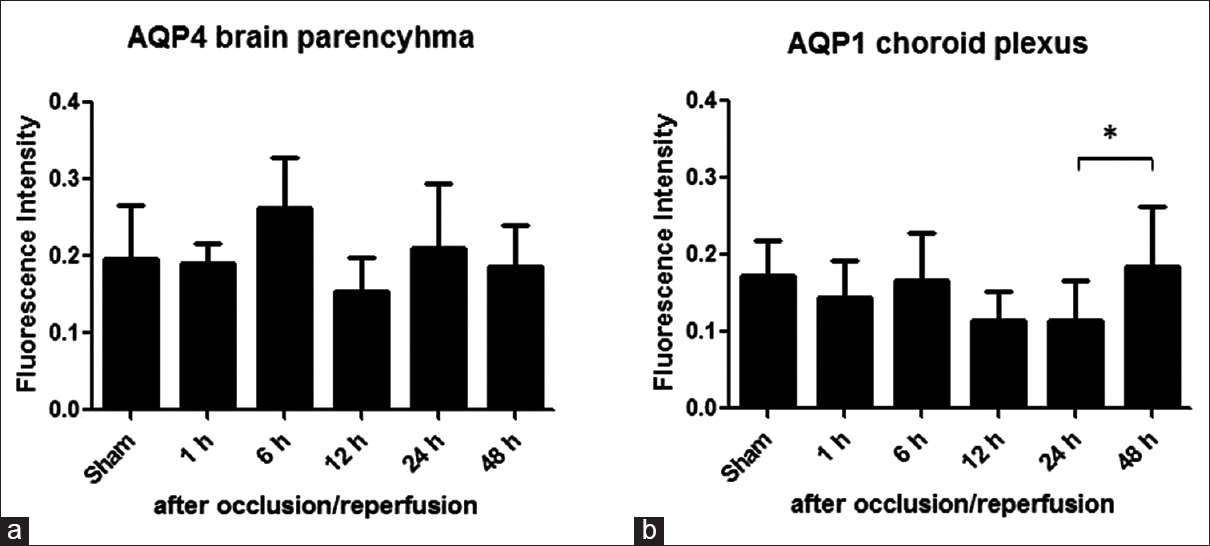
Figure 4
Aquaporins expressions: Time course change of aquaporins expression was evaluated by calculating the ratio of the fluorescence intensity of aquaporins immune-labeling in rat brain parenchyma and choroid plexus in the third ventricle. (a) The aquaporin 4 expression increase at 6 and 24 h after occlusion/reperfusion was not statistically significant. (b) However, aquaporin 1 expression increased at 48 h after occlusion/reperfusion was statistically significant (standard error, 4–6 rats/group *P < 0.05)
Western blotting of aquaporin 4
Quantification of AQP4 expression with western blot showed that change in level is not statistically significant (data not shown).
DISCUSSION
In this research, we measured AQP4 and AQP1 expression levels in brain and choroid plexus through time course after global cerebral ischemia model. We show that AQP4 expression shows statically not significant changes after ischemic brain edema. On the other hand, AQP1 expression levels showed statistically significant decrease after 24 h following ischemic brain edema.
Both carotid artery and vertebral arteries supply blood to the whole brain and choroid plexus. Four-vessel occlusion model, which consists of permanent coagulation of vertebral arteries followed by temporary occlusion of carotid arteries after 24 h, give rises to global brain ischemia and choroid plexus ischemia. An extensive neuronal damage in the caudate-putamen and paramedian and CA1 zones of the hippocampus is seen after 30 min of four-vessel occlusion. Pulsinelli et al. noted that forebrain ischemia induced by four-vessel occlusion resulted in choroid plexus necrosis at 6 h but that choroid plexus morphology returned to normal after 24 h.[
Global ischemia caused by complete interruption of cerebral blood flow results in the rapid breakdown of metabolic and electrophysiological functions of the brain. Associated edema formation has a characteristic time course.[
Vasogenic cerebral edema defined as the net extravasation of protein-rich fluid into the brain after the breakdown of the BBB. Following global ischemia, interstitial space shows expansion because of the entrance of serum proteins and fluids from the bloodstream after 6–12 h.[
Measuring wet-dry ratio of the brain is one of the major methods to quantify brain edema. Our work shows the cytotoxic and vasogenic edema model together with global cerebral ischemia model.
We histologically showed that neural injury in the hippocampal CA1 sector and dentate gyrus at 48 h after 30-min ischemia and reperfusion. Whole pyramidal cells damaged in CA1 subregion and no damage is observed in sham group.
AQP4 expression brain parenchyma and AQP1 expression in choroid plexus are measured after occlusion/reperfusion. The increase in AQP4 expression at 6 h and the following decrease at 12 h shows that cytotoxic edema is followed by vasogenic edema. A second increase at 24 may indicate the resolution of cytotoxic edema. However, none of these changes were statistically significant. It can be explained by AQP4's bidirectional role in cell membrane during brain edema as it both facilitates cellular water uptake in cytotoxic edema and clearance of extracellular fluid from in vasogenic edema. AQP1 plays a part in CSF secretion and absorption. AQP1 is expressed in the apical surface of the choroid plexus epithelium.[
Histological evaluation of CA1 neurons shrinkage in the hippocampal region shows cell necrosis and AQP4 expression in the same region is also shown with immunostaining. This indicates that neuron death. Choroid plexus region also shows cell necrosis and AQP1 expression is decreased in the selected region.
Previous studies showed that AQP1 and AQP4 expression could be targeted for edema treatment. Our studied showed that AQP4 is continuously active during ischemic edema and its expression in tissues does not change in time course. Since it has a bidirectional role while developing drugs that target AQP4, the time course of edema and type of edema must be considered.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y. Aquaporin water channels – From atomic structure to clinical medicine. J Physiol. 2002. 542: 3-16
2. Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002. 22: 367-78
3. Badaut J. Aquaglyceroporin 9 in brain pathologies. Neuroscience. 2010. 168: 1047-57
4. Chalela JA, Merino JG, Warach S. Update on stroke. Curr Opin Neurol. 2004. 17: 447-51
5. Dienel GA. Regional accumulation of calcium in postischemic rat brain. J Neurochem. 1984. 43: 913-25
6. Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab. 2008. 28: 1674-85
7. Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987. 72: 236-9
8. Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007. 22: E1-
9. Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997. 17: 171-80
10. Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993. 90: 7275-9
11. Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005. 19: 76-8
12. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013. 14: 265-77
13. Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982. 11: 491-8
14. Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979. 10: 267-72
15. Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008. 32: 200-19
16. Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007. 6: 258-68
17. Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: Reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014. 37: 620-8
18. Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: Important but elusive drug targets. Nat Rev Drug Discov. 2014. 13: 259-77