- Neuronursing Division, National Institute of Nursing Education, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Psychiatric Nursing Division, College of Nursing, All India Institute of Medical Sciences, New Delhi, India
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Sivashanmugam Dhandapani
Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India
DOI:10.4103/2152-7806.179229
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dhandapani M, Gupta S, Mohanty M, Gupta SK, Dhandapani S. Trends in cognitive dysfunction following surgery for intracranial tumors. Surg Neurol Int 22-Mar-2016;7:
How to cite this URL: Dhandapani M, Gupta S, Mohanty M, Gupta SK, Dhandapani S. Trends in cognitive dysfunction following surgery for intracranial tumors. Surg Neurol Int 22-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/trends-in-cognitive-dysfunction-following-surgery-for-intracranial-tumors/
Abstract
Background:This study was conducted to prospectively assess the cognitive function of patients with intracranial tumors.
Methods:The cognitive status of patients with intracranial tumors were prospectively studied before surgery, and later at 1 and 6 months following surgery, on purposive sampling, using validated post graduate institute (PGI) battery for brain dysfunction (score 0–30) with a higher dysfunction rating score indicating poor cognitive status.
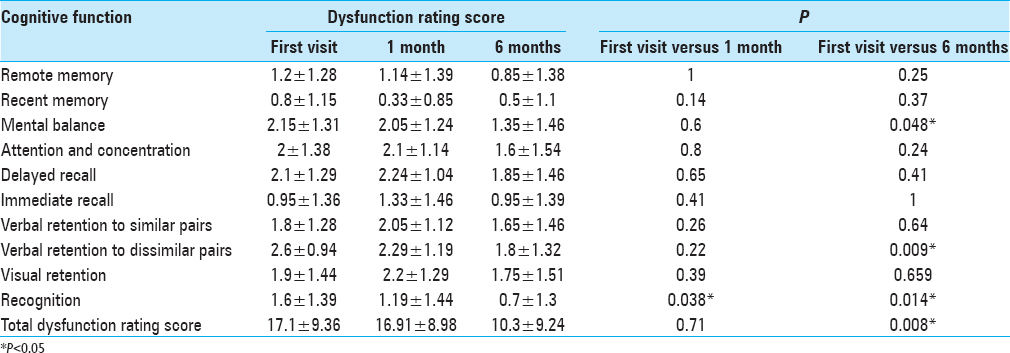
Results:Out of 23 patients enrolled, 20 could complete the study. They had substantial cognitive dysfunction before surgery (score 17.1 ± 9.4). Though there was no significant improvement (16.9 ± 9.0) at 1 month, the score improved significantly (10.3 ± 9.2) at 6 months following surgery (P = 0.008). The improvement was relatively subdued in intra-axial, malignant, and radiated tumors. Overall, there was a significant improvement in mental balance (P = 0.048), verbal retention of dissimilar pairs (P = 0.01), and recognition (P = 0.01), while dysfunction persisted in the domains of memory, verbal retention to similar pairs, and visual retention.
Conclusion:Patients with intracranial tumors have substantial cognitive dysfunction, which tend to show significant improvement beyond 6 months following surgery, especially among tumors, which were extra-axial, benign, and nonirradiated.
Keywords: Cognitive dysfunction, intracranial tumor, memory scales, surgery
INTRODUCTION
Intracranial tumors are one of the common causes of mortality and morbidity worldwide.[
Cognitive dysfunction in these patients can impair the intellectual functions, activities of daily living (ADL), interpersonal relationships, and education and profession of the patients. It may prevent them from fulfilling their familial, professional, and social obligations thus can lead to poor quality of life (QoL) not only in patients,[
The factors leading to cognitive deficits include paraneoplastic effects such as duration and location of the tumor,[
There is lack of evidence on the domains, magnitude, and duration of cognitive dysfunction present in patients with intracranial tumors. Cognitive deficits of these patients are neither monitored nor managed during the follow-up visits. Knowing the trend in cognitive dysfunction is necessary to develop protocols and tools for assessing cognitive function. Identifying the magnitude and type of cognitive dysfunction, may aid in various cognitive behavioral therapies to improve the QoL of patients and caregivers. Appropriate interventions can be developed based on the specific cognitive impairment of the patients. However, there is a paucity of studies on trends in cognitive dysfunction with respect to the treatment provided. With this in mind, this study was undertaken to assess the cognitive function of the patients with intracranial tumors before surgery, later at 1 month and 6 months following surgery.
MATERIALS AND METHODS
A longitudinal study was done to assess the cognitive changes in patients with intracranial tumors. Using purposive sampling, 23 newly diagnosed conscious intracranial tumor patients who were availing treatment from a Tertiary Care Center at Chandigarh and consented to the study were enrolled. Ethical clearance was obtained from the Institute Ethics Committee, and written consent was taken from all study subjects and guardians. All patients who underwent surgical management with or without radiotherapy or chemotherapy were followed up for cognitive assessment. Cognitive status was assessed before surgery, later at 1 month and 6 months following surgery.
Cognitive changes of patients were assessed using PGI battery for brain dysfunction (BBD) which assesses various domains of cognitive functions.[
The raw score of each domain was made into a converted score according to the educational level of the patients. The total dysfunction score was calculated by adding the dysfunction scores of all domains and higher the dysfunction rating score poorer the cognitive status. The total maximum possible score is 30 (3 in each domain).
SPSS 21 (IBM, New York, USA) was utilized for descriptive and inferential statistics. Repeated measures ANOVA was used to compare the dysfunction score at different assessment.
RESULTS
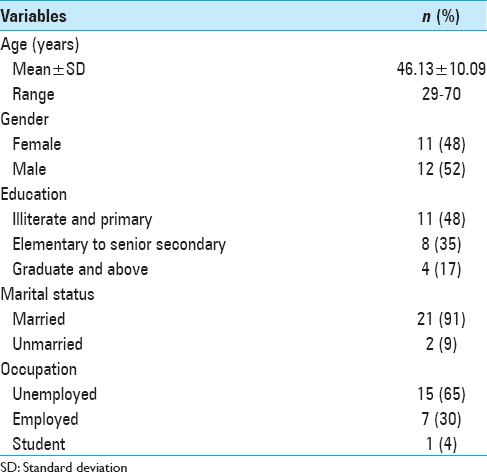
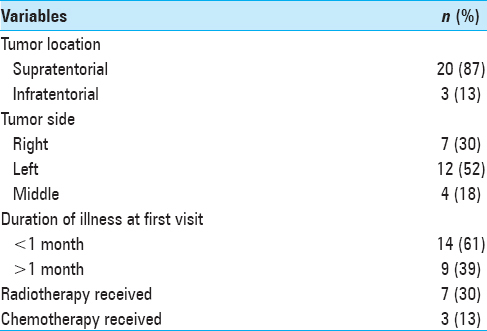
Out of 23 patients with intracranial tumors enrolled, 20 patients were followed up as 2 patients died, and 1 patient was lost to follow-up. As per the sociodemographic data of patients shown in
Cognitive changes in patients
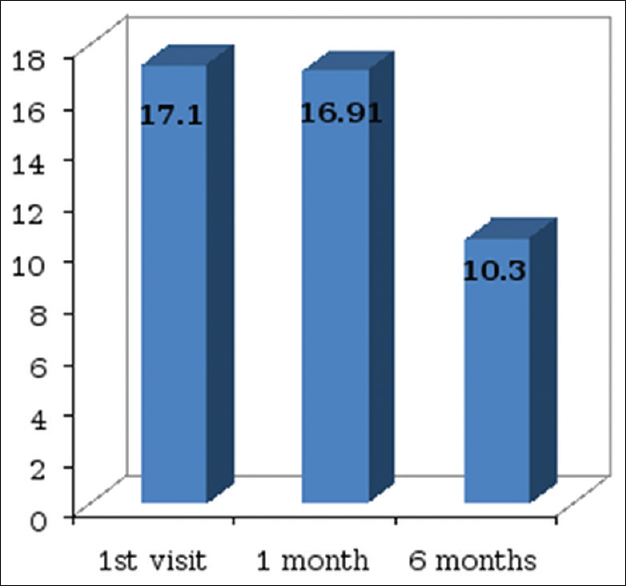
The cognitive function of the patients was significantly affected as the cognitive dysfunction score was 17.1 ± 9.4 out of 30 before any definite treatment (higher the score higher the dysfunction). There was an only mild nonsignificant improvement in the dysfunction rating score (16.91 ± 9.0) at 1 month of follow-up. There was a significant improvement only in recognition (P = 0.04).
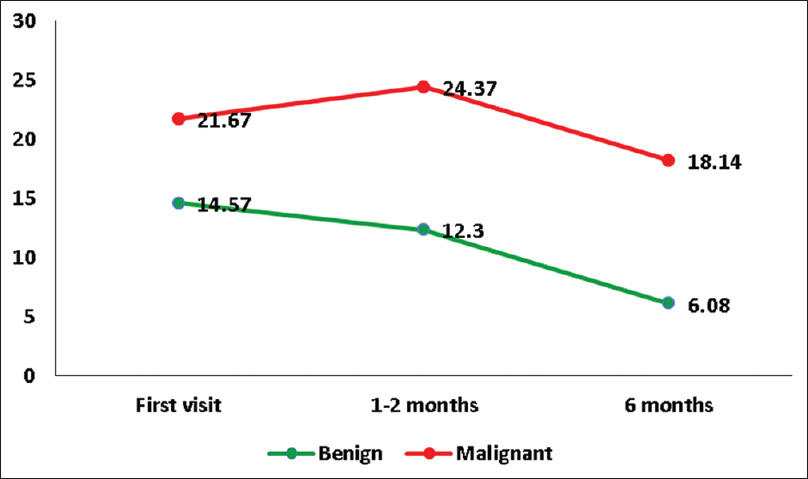
There was a significant improvement in cognitive function at 6 months with the dysfunction rating score decreasing from 17.1 ± 9.4 to 10.3 ± 9.2 (P = 0.01) [
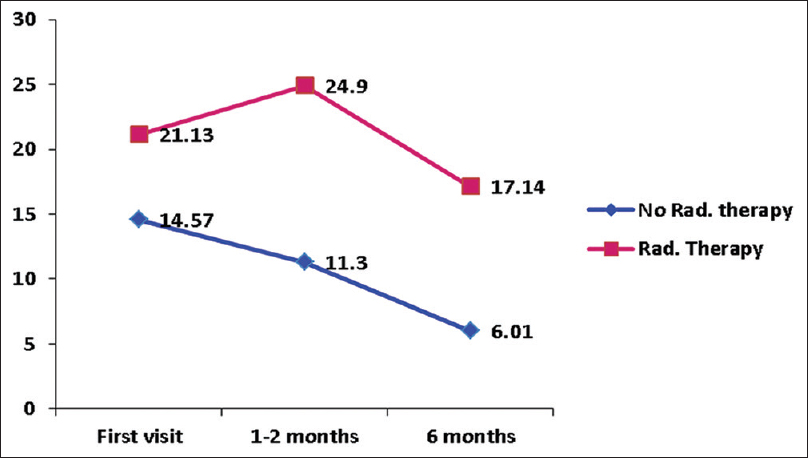
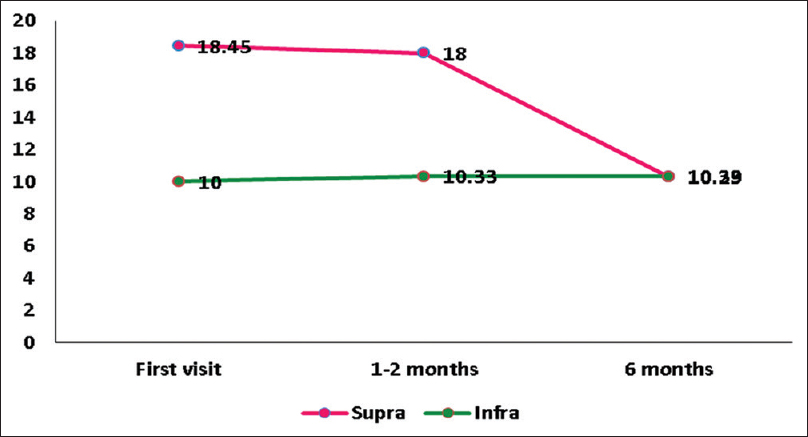
As shown in Figures
DISCUSSION
Cognitive dysfunction in patients with intracranial tumors is well evident in literature.[
There are many domains of cognitive function. There is no conclusive evidence on the particular domain being affected due to the difference in the cognitive tools used in various studies. In this study, most affected by all domains on first assessment was verbal retention to dissimilar pairs, an indicator of new learning. Other domains highly affected were a mental balance, attention, concentration, and delayed recall. The domains least affected were a recent memory and immediate recall. Cognitive deficits reported in literature include deficits in working memory, cognitive control, cognitive processing speed, visual searching, planning foresight, general attention,[
Cognitive deficits are reported in these patients before any definitive treatment, at 3 months postradiotherapy,[
The overall cognitive function at 6 months following surgery was significantly improved (P = 0.008). Though there was an improvement of cognitive function in all domains at 6 months, mental balance, verbal retention of dissimilar pairs and recognition have shown significant improvement [
The cognitive deficits identified in this study before initiation of radiotherapy, chemotherapy, or surgery explains the influence of tumor and its pathological process on cognitive deficits. It is reported that patients with frontal glioma exhibit more cognitive deficits due to the damage in the frontal lobe.[
Cognitive function was found to be better in patients with benign tumor and poor in patients who underwent radiotherapy at all points of assessment. The majority of the patients who received radiotherapy had a malignant tumor. The aggressive nature of the tumor along with radiotherapy can result in higher cognitive dysfunction. A mild deterioration in cognitive function at 1 month observed in patients of the malignant tumor could be due to the transient neurotoxic effect of radiation.[
Cognitive dysfunction leads to difficulties in performing cognitive tasks, contribute to functional disability, and behavioral changes and thus, makes the individual handicapped.[
This study is limited because of small sample size. Multicenter studies of larger sample size are needed to identify the significant relationship of various factors described in this study. A long-term follow-up of cognitive function will aid in cognitive rehabilitation which can be individualized for the patients with and without improvement in the cognitive function. As the cognitive dysfunction was found persisting even at 6 months, monitoring cognitive task, and developing appropriate management strategies are important to equip these patients in their personal, as well as social life. The neuro-oncology team must facilitate this to improve QoL for patients and their caregivers. Socioeconomic barriers are reported to result in irregular follow-up visits and poor outcome among neurosurgical patients.[
CONCLUSION
Patients with intracranial tumors have substantial cognitive dysfunction before any definite treatment. However, there was a significant improvement in cognitive function at 6 months following surgery with deficits persisting in some domains. One of the long-term goals for the neuro-oncology team must be to reduce the cognitive sequelae of these tumors with improvement in treatment modalities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Armstrong CL, Hunter JV, Ledakis GE, Cohen B, Tallent EM, Goldstein BH. Late cognitive and radiographic changes related to radiotherapy: Initial prospective findings. Neurology. 2002. 59: 40-8
2. Arreguín González IJ, Galicia RD, Yánez G, Edgardo R, Luviano VL, Hernández L. Neuropsychologic evaluation in a patient with a frontal meningioma. Arch Neurociencias. 2008. 13: 59-68
3. Bunevicius A, Tamasauskas S, Deltuva V, Tamasauskas A, Radziunas A, Bunevicius R. Predictors of health-related quality of life in neurosurgical brain tumor patients: Focus on patient-centered perspective. Acta Neurochir (Wien). 2014. 156: 367-74
4. Correa DD, Shi W, Thaler HT, Cheung AM, DeAngelis LM, Abrey LE. Longitudinal cognitive follow-up in low-grade gliomas. J Neurooncol. 2008. 86: 321-7
5. Dhandapani M, Gupta S, Dhandapani S, Kaur P, Samra K, Sharma K. Study of factors determining caregiver burden among primary caregivers of patients with intracranial tumors. Surg Neurol Int. 2015. 6: 160-
6. Dhandapani S, Karthigeyan M, Dhandapani M. Unusual presentation of hyperphagia after surgery for fourth ventricular ependymoma: Accessory satiety center or hypothalamic seedlings?. Neurol India. 2013. 61: 320-1
7. Dhandapani S, Sharma A, Sharma K, Das L. Comparative evaluation of MRS and SPECT in prognostication of patients with mild to moderate head injury. J Clin Neurosci. 2014. 21: 745-50
8. Dhandapani S, Sharma K. Is “en-bloc” excision, an option for select large vascular meningiomas?. Surg Neurol Int. 2013. 4: 102-
9. Dhandapani SS, Manju D, Mahapatra AK. The economic divide in outcome following severe head injury. Asian J Neurosurg. 2012. 7: 17-20
10. Dwarka P, Santosh KV.editorsHandbook of PGI Battery of Brain Dysfunction (PGI-BBD). Agra: National Psychological Corporation, Modern Printers; 1990. p. 172-
11. Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the childhood cancer survivor study. Neuropsychology. 2009. 23: 705-17
12. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995. 163: 19-25
13. Fliessbach K, Helmstaedter C, Urbach H, Althaus A, Pels H, Linnebank M. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: A prospective study. Neurology. 2005. 64: 1184-8
14. Gehrke AK, Baisley MC, Sonck AL, Wronski SL, Feuerstein M. Neurocognitive deficits following primary brain tumor treatment: Systematic review of a decade of comparative studies. J Neurooncol. 2013. 115: 135-42
15. Horská A, Laclair A, Mohamed M, Wells CT, McNutt T, Cohen KJ. Low cerebellar vermis volumes and impaired neuropsychologic performance in children treated for brain tumors and leukemia. AJNR Am J Neuroradiol. 2010. 31: 1430-7
16. Howlader NNoone AMKrapcho MGarshell JMiller DAltekruse SFLast accessed on 2015 Jul 08. Available from: http://www.seer.cancer.gov/csr/1975_2011/ .
17. Kangas M, Tate RL, Williams JR, Smee RI. The effects of radiotherapy on psychosocial and cognitive functioning in adults with a primary brain tumor: A prospective evaluation. Neuro Oncol. 2012. 14: 1485-502
18. Koizumi H, Ideguchi M, Iwanaga H, Shirao S, Sadahiro H, Oka F. Cognitive dysfunction might be improved in association with recovered neuronal viability after intracranial meningioma resection. Brain Res. 2014. 1574: 50-9
19. Lageman SK, Cerhan JH, Locke DE, Anderson SK, Wu W, Brown PD. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. J Neurooncol. 2010. 96: 271-6
20. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009. 11: 330-9
21. Manoharan N, Julka PK, Rath GK. Descriptive epidemiology of primary brain and CNS tumors in Delhi, 2003-2007. Asian Pac J Cancer Prev. 2012. 13: 637-40
22. Marsh JC, Gielda BT, Herskovic AM, Abrams RA. Cognitive sparing during the administration of whole brain radiotherapy and prophylactic cranial irradiation: Current concepts and approaches. J Oncol 2010. 2010. p.
23. Martens T, Rotermund R, Zu Eulenburg C, Westphal M, Flitsch J. Long-term follow-up and quality of life in patients with intracranial germinoma. Neurosurg Rev. 2014. 37: 445-50
24. Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998. 16: 2522-7
25. Mu YG, Huang LJ, Li SY, Ke C, Chen Y, Jin Y. Working memory and the identification of facial expression in patients with left frontal glioma. Neuro Oncol. 2012. 14: iv81-9
26. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004. 5: 399-408
27. Neisser U.editorsCognitive Psychology. Englewood Cliffs: Prentice-Hall; 1967. p.
28. Ory MG, Hoffman RR, Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999. 39: 177-85
29. Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001. 19: 2302-8
30. Poggi G, Liscio M, Galbiati S, Adduci A, Massimino M, Gandola L. Brain tumors in children and adolescents: Cognitive and psychological disorders at different ages. Psychooncology. 2005. 14: 386-95
31. Prabhu RS, Won M, Shaw EG, Hu C, Brachman DG, Buckner JC. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: Secondary analysis of RTOG 98-02. J Clin Oncol. 2014. 32: 535-41
32. Satoer D, Vork J, Visch-Brink E, Smits M, Dirven C, Vincent A. Cognitive functioning early after surgery of gliomas in eloquent areas. J Neurosurg. 2012. 117: 831-8
33. Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008. 70: 826-34
34. Sherwood PR, Given BA, Doorenbos AZ, Given CW. Forgotten voices: Lessons from bereaved caregivers of persons with a brain tumour. Int J Palliat Nurs. 2004. 10: 67-75
35. Surma-aho O, Niemelä M, Vilkki J, Kouri M, Brander A, Salonen O. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001. 56: 1285-90
36. Taylor BV, Buckner JC, Cascino TL, O’Fallon JR, Schaefer PL, Dinapoli RP. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol. 1998. 16: 2195-201
37. van der Vossen S, Schepers VP, Berkelbach van der Sprenkel JW, Visser-Meily JM, Post MW. Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med. 2014. 46: 430-7
38. Welzel G, Steinvorth S, Wenz F. Cognitive effects of chemotherapy and/or cranial irradiation in adults. Strahlenther Onkol. 2005. 181: 141-56
39. Zucchella C, Bartolo M, Di Lorenzo C, Villani V, Pace A. Cognitive impairment in primary brain tumors outpatients: A prospective cross-sectional survey. J Neurooncol. 2013. 112: 455-60