- Department of Neurosurgery, Hospital of Restoration-University of Pernambuco, Recife, Brazil
- Department of Pediatric Neurosurgery, IMIP, Recife, Brazil
- Department of Neurosurgery, Universitary Hospital, Federal University of Vale do São Francisco, Petrolina, Brazil
- Department of Pediatric Neurosurgery, IMIP, Petrolina, Brazil
Correspondence Address:
Ricardo Brandão Fonseca
Department of Neurosurgery, Hospital of Restoration-University of Pernambuco, Recife, Brazil
DOI:10.4103/2152-7806.170540
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Faquini I, Ricardo Brandão Fonseca, Sérgio Luís Vale de Melo, Negri H, Vieira E, Saboia T, Azevedo-Filho H. Trigone ventricular meningiomas: Is it possible to achieve good results even in the absence of high tech tools?. Surg Neurol Int 26-Nov-2015;6:180
How to cite this URL: Faquini I, Ricardo Brandão Fonseca, Sérgio Luís Vale de Melo, Negri H, Vieira E, Saboia T, Azevedo-Filho H. Trigone ventricular meningiomas: Is it possible to achieve good results even in the absence of high tech tools?. Surg Neurol Int 26-Nov-2015;6:180. Available from: http://surgicalneurologyint.com/surgicalint_articles/trigone-ventricular-meningiomas-is-it-possible-to-achieve-good/
Abstract
Background:Intraventricular meningiomas constitute 2% of intracranial meningiomas, representing a challenging disease for neurosurgeons; we report our experience through a case series, emphasizing surgical approaches and results.
Methods:Between 2009 and 2012, four patients underwent microsurgical resection in our department. Clinical and imaging findings, surgical approaches, outcomes, and follow-up were analyzed.
Results:Four patients (three females and one male) were included and the signs of intracranial hypertension were the main clinical presentation in all cases. The parietal approach through intraparietal sulcus was performed in 3 cases and parieto-occipital interhemispheric surgical route in 1 case. Gross total resection was achieved in all the patients without additional deficits and without the aid of neuronavigation, intraoperative monitoring, and intraoperative magnetic resonance imaging.
Conclusion:Gross total resection is the gold standard treatment for such tumors and the intraparietal sulcus approach is an excellent choice for most of the cases. Careful anatomical knowledge contributes to a safer procedure even in the absence of high tech equipment assistance.
Keywords: Lateral ventricle, meningioma, trigone
BACKGROUND
Intraventricular meningiomas are uncommon lesions which represent about 0.5–5% (average 2%) of all meningiomas. The most common location is atrioventricular.[
Objectives
Atrioventricular meningiomas are very rare lesions. We present 4 cases with a literature review of the surgical decision process, with an emphasis on approaches and management issues as well as outcomes. Also, we report the experience of our department with four meningiomas of ventricular trigone operated without neuronavigation, intraoperative monitoring, and intraoperative magnetic resonance imaging (MRI) and discuss the most suitable surgical approach based on literature.
METHODS
A retrospective review of four patients who underwent a surgical operation at a reference center in neurosurgery (Hospital of Restoration - Recife-PE) between April 2009 and March 2013 [
REPORTS
Case 1
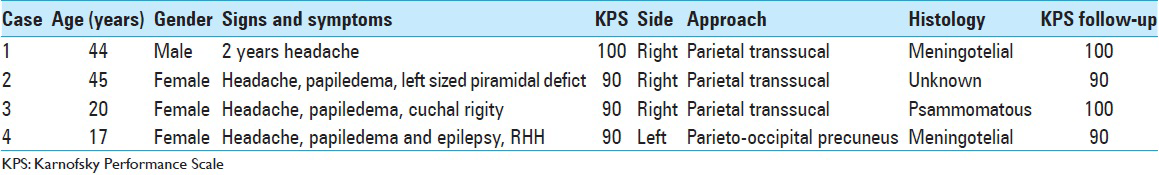
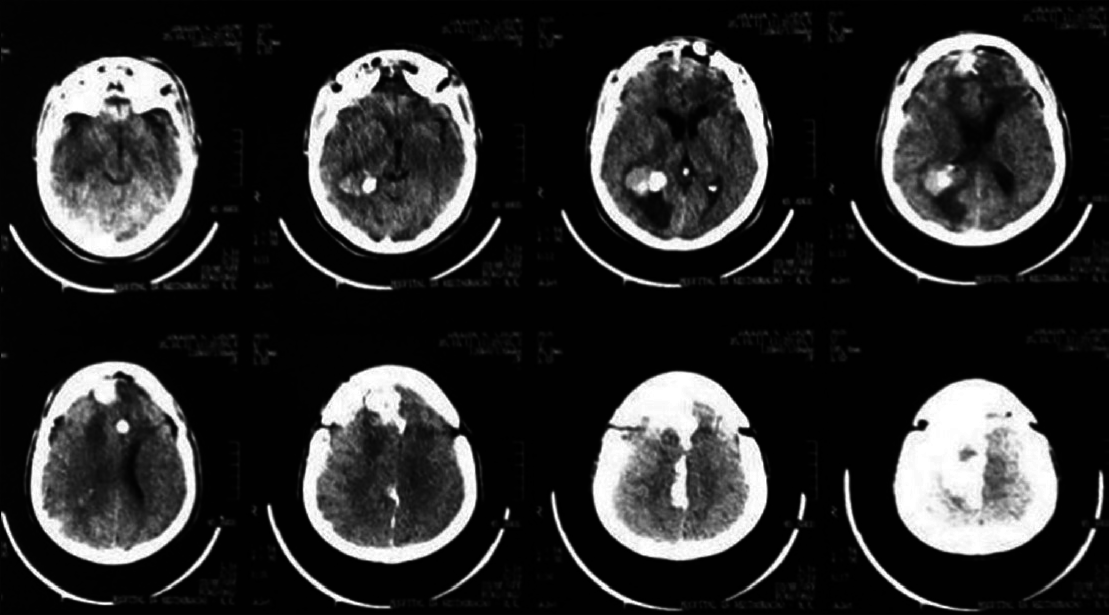
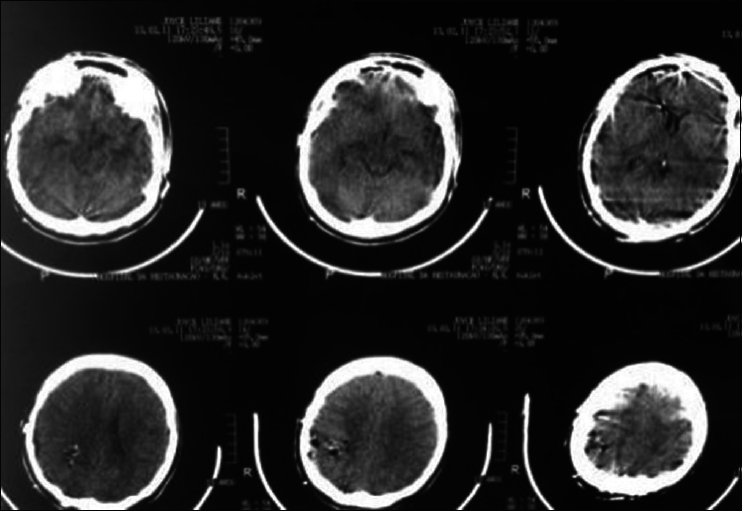
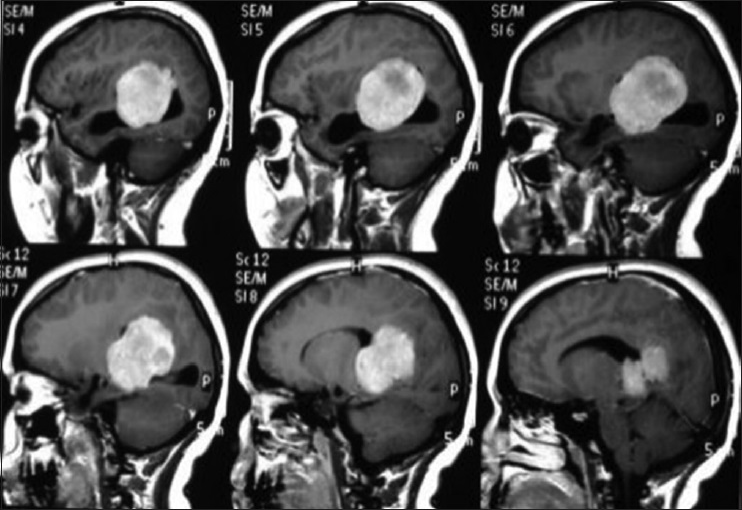
A 44-year-old male patient presented with morning headache and no other complains at the emergency room. Physical examination showed no neurological deficits. The computed tomography (CT) scan revealed a hyperdense lesion with homogeneous and intense contrast enhancement in the right ventricular trigone. The patient was submitted to surgery and placed supine with the head in neutral position fixed with Mayfield. A “C” shaped incision was performed and followed by a craniotomy of 6 cm lateral to the midline and 8 cm anterior to lambda. A dural opening with the base facing the superior sagital sinus was made. At this point, the intraparietal sulcus was identified running parallel and 3 cm lateral to the midline. Trans-sulcal dissection was performed toward the ventricular atrium's roof. A straight line direction is fundamental due to the fact that optical radiation fibers are laterally and inferiorly localized. Tumor debulking and resection was achieved without the brain retraction. In postoperative, no new deficits were added and the patient remains asymptomatic after a 3 years follow-up without signs of recurrence [Figures
Case 2
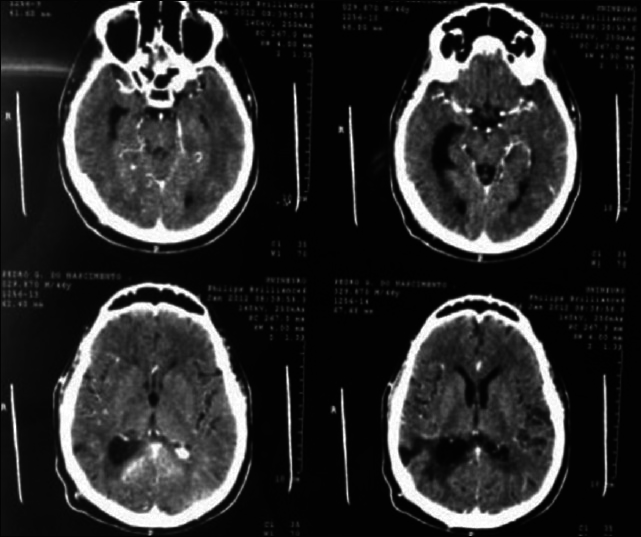
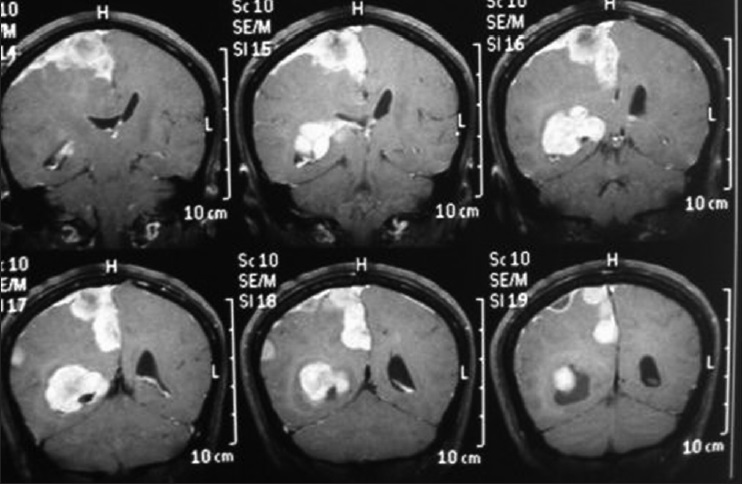
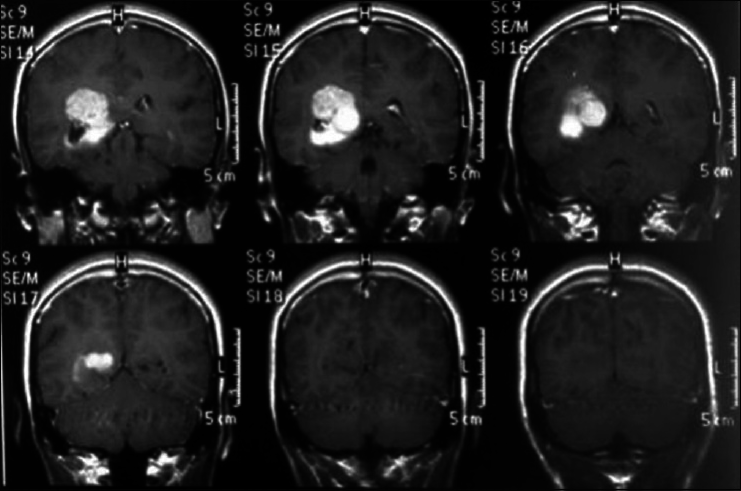
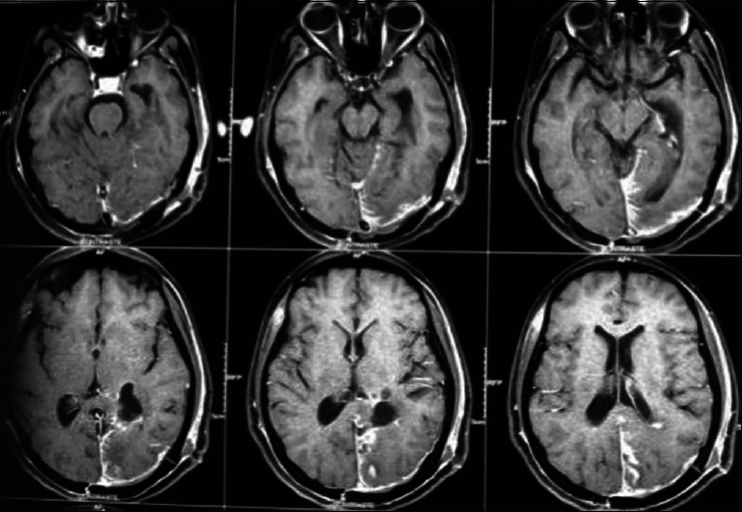
A 45-year-old female presented with a headache and difficulty walking. On examination, she had papilledema and a left sized pyramidal motor syndrome. CT scan revealed multiple meningiomas. The first approach was directed to the parasagittal lesion and the second surgery to the atrium tumor. She underwent the craniotomy with a parietal approach through the intraparietal sulcus. Complete resection of both lesions was achieved with the clinical improvement and no additional deficits [
Axial T2 MRI sequence is showing multiple meningiomas with calcifications and perilesional vasogenic edema [Figures
Case 3
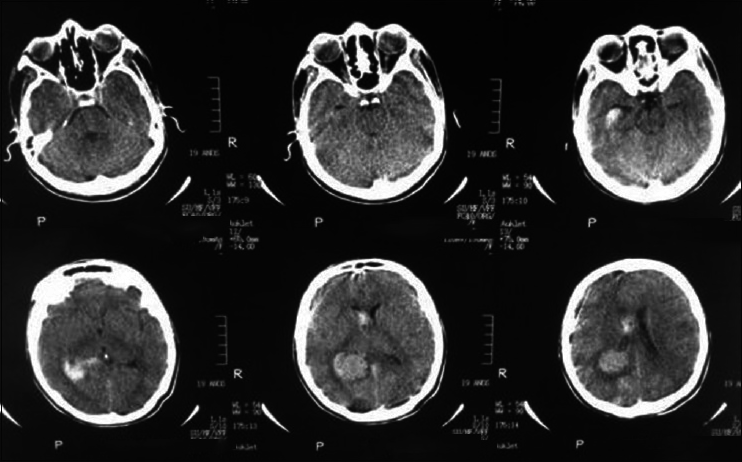
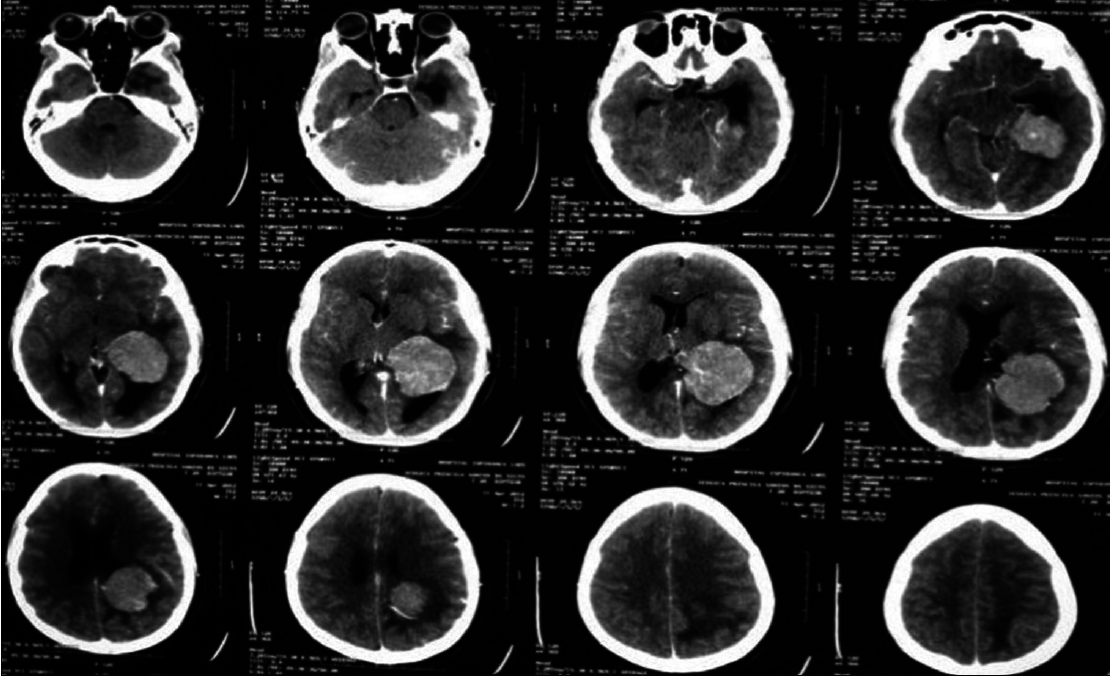
A 20-year-old female patient presented with a headache and vomiting. On examination, she had neck stiffness and papilledema. CT scan showed a large tumor in the right ventricular atrium. The same surgical approach was used and a total resection of the lesion was achieved. She was discharged in 3 days after the surgery without additional deficits and no recurrence after 2 years follow-up [Figures
Case 4
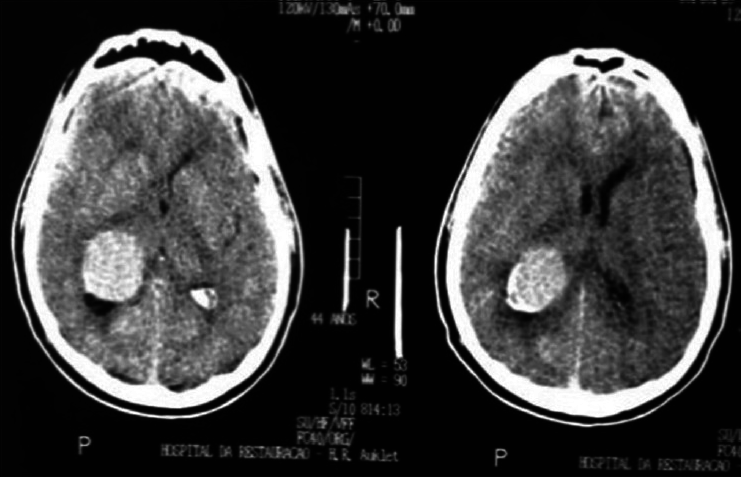
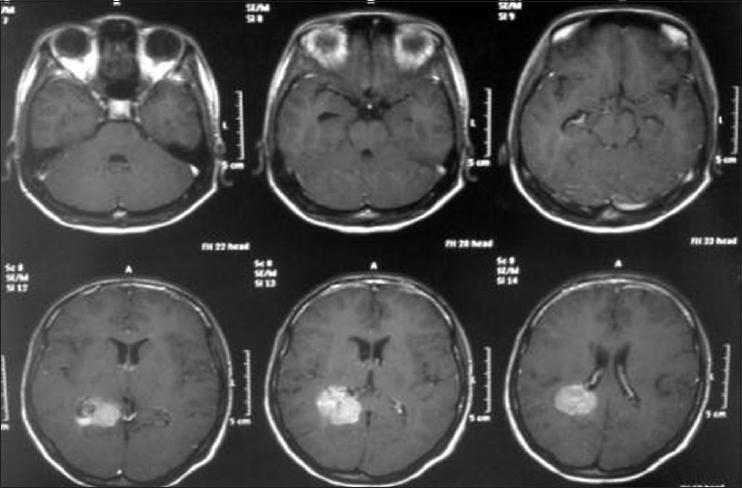
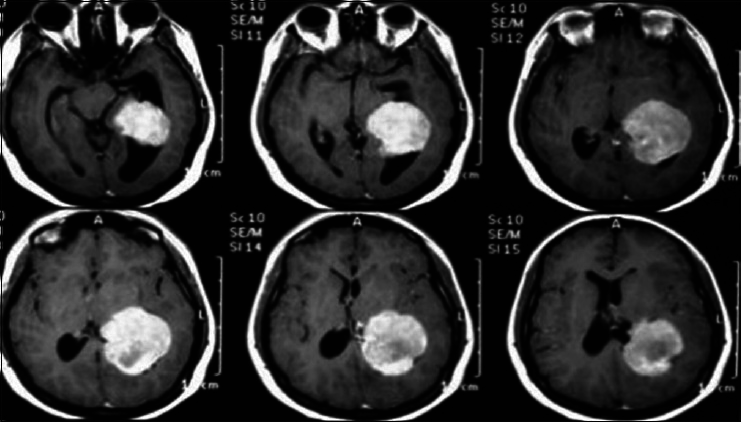
A 17-year-old female patient presented with a headache associated with the blurred vision and two previous seizures. On examination, right homonymous hemianopsia and papilledema were evident. CT scan showed a hyperdense lesion in the left ventricular trigone and underlying vasogenic edema. She underwent a surgical operation and the parieto-occipital interhemispheric precuneus approach (paraesplenial) was elected due to the fact that the tumor was in contact with the medial surface of the brain. In this case, the patient was operated on ¾ prone with the head parallel to the ground. Tumor side (left side) down facilitated resection aided by gravity. Gross total resection was obtained without the additional deficits. The patient remained with right homonymous hemianopia postoperatively [Figures
RESULTS
In this case series, all the patients had large lesions (>3 cm) with an average age of 31.5 and female sex prevalence. Contrary to what we found in literature, there is a predominance of lesions on the right side (three). Signs or symptoms of intracranial hypertension occurred in four patients. A headache was reported in all the patients and three had papilledema on physical examination. Only one patient had visual field disturbance (homonymous hemianopsia). Also, only one patient presented a prior pyramidal deficit syndrome and another one, a history of seizures.
With regards to the surgical approach, 3 cases were approached through a parietal route along the intraparietal sulcus and one by a parieto-occipital interhemispheric precuneus approach. All the patients had tumor gross total resection without the additional morbidity and improvement of preoperative Karnofsky Performance Scale was achieved in 2 cases.
DISCUSSION
Intraventricular meningiomas are uncommon lesions accounting for 0.5–2% of intracranial meningiomas and their commonest location is in the ventricular trigone.
Among intraventricular meningiomas, 77–90% is found in the atrium ventricular region. They also represent 9.8–14% of all ventricular tumors and 20% of all tumors inside the lateral ventricle. The first atrial ventricular meningioma was described by Schaw et al. (1853)[
These tumors are located deep in the brain, with surrounding intact brain tissue near vital ventricular structures and are vascularized by the anterior and posterior lateral choroidal artery. Lenticulostriate arteries and also participates in tumor's irrigation[
Atrioventricular meningiomas are resistant to the nonsurgical treatment. Radiosurgery is reserved only for the lesions smaller than 2 cm and can be used for residual or recurrent.[
With the objective of maximum tumor resection with less surgical morbidity, the anatomical knowledge through craniometric points and surgical safe corridors through the brain are essential, especially in developing countries like ours where technological assistance features such as neuronavigation and intraoperative monitoring, are not available at all hospitals due to the high cost. This craniometric knowledge should be taught and still practiced during residency, thus enabling the future neurosurgeon confidence and security needed to obtain good results even in the absence of high tech equipment.
Different approaches to these tumors were described in the literature making it a difficult task for neurosurgeons to elect the best for each case. On this note, one must consider that the aim must be a good exposure of the lesion and also an early visualization of the arterial pedicle. The main purpose is to accomplish complete removal with minimal or no damage to surrounding brain tissue. The principal approaches in literature are: Superior parietal transcortical approach (trans-sulcal), temporal approach (middle temporal gyrus), parieto-occipital interhemispheric approach (paresplenial), subtemporal, transtentorial supracerebellar, contralateral transfalcine, and posterior transcallosal approach. Cramer et al. (1960)[
Kempe and Blaylock (1976)[
Suitable for small lesions of the midline and not requires the strong hemispheric retraction, this approach offers a quick access to branches of the posterior choroidal artery and medial atrial vein. Yasargil et al. (1996)[
To avoid the brain retraction, it is necessary to carry out debulking and piecemeal resection.
Thereafter, the tumor capsule is deflected, exposing the arterial pedicle, and giving the possibility to coagulation. The ultrasonic aspirator is extremely useful. It is imperatively rigorous for hemostasis to leave an external ventricular drainage postoperatively to prevent an acute hydrocephalus.[
CONCLUSION
Careful surgical planning and knowledge of anatomy is essential for a good outcome, especially in the absence of high tech tools. We consider the parietal approach through the intraparietal sulcus as the best choice for virtually all cases, considering that it provides a straight line pathway to lateral ventricle's trigone, in the shortest route and avoids the neurological morbidity to the patient. This approach was performed successfully in 3 out of 4 cases without the aid of neuronavigation, intraoperative monitoring, and intraoperative MRI. The parieto-occipital interhemispheric transprecuneus approach is an alternative choice, especially for those cases with a medial projection of the lesion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cramer F. The intraventricular meningiomas: a note of the neurologic determinants governing the surgical approach. Arch Neurol. 1960. 3: 98-
2. Criscuolo GR, Symon L. Intraventricular meningioma. A review of 10 cases of the National Hospital, Queen Square (1974-1985) with reference to the literature. Acta neurochirurgica. 1986. 83: 83-91
3. Cushing H, Eisenhardt L.editors. Meningiomas: their classification, regional behaviour, life history and surgical end results. Springfield, Illinois: Charles C. Thomas; 1938. p.
4. D’Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lat-eral ventricle tumors: surgical strategies according to tumor origin and de-velopment - a series of 72 cases. Neurosurgery. 2005. 56: S36-S45
5. Delandsheer JM. Meningiomas of the Lateral Ventricle. Neuro-Chirurgie. 1965. 11: 3-83
6. Dreifus W. Ueber endothelium des plexus choroideus. Beitr Path Anat. 1923. 71: 667-673
7. Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001. 10: 1-13
8. Fornari M, Savoiardo M, Morello G, Solero L. Meningiomas of the lateral ventricles. Neuroradiological and surgical considerations in 18 cases. J Neurosurg. 1981. 54: 64-74
9. Kempe LG, Blaylock R. Lateral-trigonal intraventricular tumors. A new operative approach. Acta neurochirurgica. 1976. 35: 233-42
10. Liu M, Liu Y, Zhu S, Li X. Intraventricular meningiomas: a report of 25 cases. Neurosurg Rev. 2006. 29: 36-40
11. MacDowall TW. Large calcareous tumor involving chiefly the inner and middle portions of the left temporosphenoidal lobe and pressing upon the left crus and optic thalamus (brief communication). Edinburgh Med J. 1881. 26: 1088-
12. Nakamura M, Roser F, Bundschuh O, Vorkapic P, Samii M. Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol. 2003. 59: 491-504
13. Olivecrona H, Tönnis W.editorsHandbuch der neurochirurgie. Berlin, Heidelberg, New York: Springer; 1967. 4: 175-77
14. Schaw A. Fibrous tumour in the lateral ventricle of the brain. Boney deposits in the arachnoid membrane of the right hemisphere. Trans Pathol Soc London. 1853. 5: 18-21
15. Yasargil MG, Yasargil MG.editors. Parieto-occipital interhemispheric approach. Microneurosurgery. New York: Thieme; 1996. IVB: 56-7






Stephen J. Seligman
Posted June 14, 2016, 8:14 pm
Could you ask Dr. Ricardo Brandão Fonseca if has the email address of Dra Maria Lucia Brito. She reported cases of ADEM secondary to Zika virus infection and we would like to set up a collaborative study with her.
jim
Posted June 14, 2016, 8:57 pm
Dr Seligman,
If you download the PDF for this article, you will find Dr Fonseca’s e-mail address in the authors’ contact information.
Best regards,
Jim Cook
Managing Editor
Surgical Neurology International