- Department of Neurosurgery, New York University School of Medicine, New York, USA
- Department of Radiology, New York University School of Medicine, New York, USA
- Department of Medicine, New York University School of Medicine, New York, USA
- Department of Pathology, New York University School of Medicine, New York, USA
- Department of Otolaryngology-Head and Neck Surgery, New York University School of Medicine, New York, USA
- Department of Perlmutter Cancer Center, New York University School of Medicine, New York, USA
- Department of Brain Tumor Center, New York University School of Medicine, New York, USA
Correspondence Address:
Richard Lebowitz, Dimitris G. Placantonakis
Department of Neurosurgery, New York University School of Medicine, New York, USA
Department of Perlmutter Cancer Center, New York University School of Medicine, New York, USA
Department of Brain Tumor Center, New York University School of Medicine, New York, USA
DOI:10.4103/sni.sni_192_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: James Barger, Matthew Siow, Michael Kader, Katherine Phillips, Girish Fatterpekar, David Kleinberg, David Zagzag, Chandranath Sen, John G. Golfinos, Richard Lebowitz, Dimitris G. Placantonakis. The posterior nasoseptal flap: A novel technique for closure after endoscopic transsphenoidal resection of pituitary adenomas. 14-Feb-2018;9:32
How to cite this URL: James Barger, Matthew Siow, Michael Kader, Katherine Phillips, Girish Fatterpekar, David Kleinberg, David Zagzag, Chandranath Sen, John G. Golfinos, Richard Lebowitz, Dimitris G. Placantonakis. The posterior nasoseptal flap: A novel technique for closure after endoscopic transsphenoidal resection of pituitary adenomas. 14-Feb-2018;9:32. Available from: http://surgicalneurologyint.com/surgicalint-articles/the-posterior-nasoseptal-flap-a-novel-technique-for-closure-after-endoscopic-transsphenoidal-resection-of-pituitary-adenomas/
Abstract
Background:While effective for the repair of large skull base defects, the Hadad-Bassagasteguy nasoseptal flap increases operative time and can result in a several-week period of postoperative crusting during re-mucosalization of the denuded nasal septum. Endoscopic transsphenoidal surgery for pituitary adenoma resection is generally not associated with large dural defects and high-flow cerebrospinal fluid (CSF) leaks requiring extensive reconstruction. Here, we present the posterior nasoseptal flap as a novel technique for closure of skull defects following endoscopic resection of pituitary adenomas. This flap is raised in all surgeries during the transnasal exposure using septal mucoperiosteum that would otherwise be discarded during the posterior septectomy performed in binostril approaches.
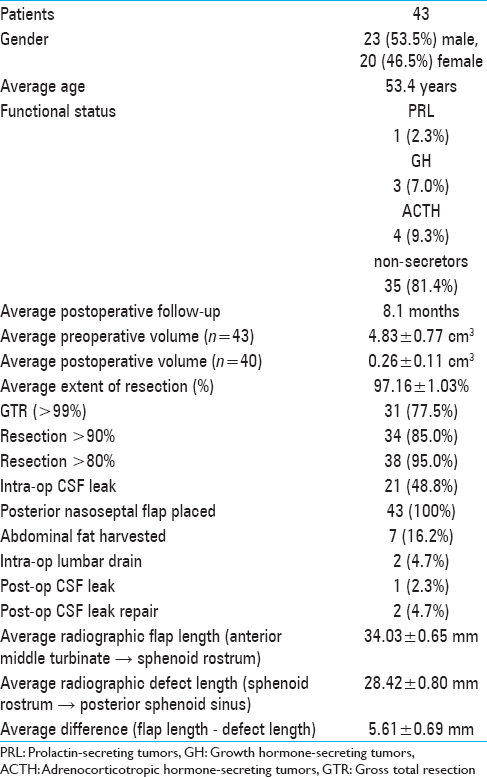
Methods:We present a retrospective, consecutive case series of 43 patients undergoing endoscopic transsphenoidal resection of a pituitary adenoma followed by posterior nasoseptal flap placement and closure. Main outcome measures were extent of resection and postoperative CSF leak.
Results:The mean extent of resection was 97.16 ± 1.03%. Radiographic measurement showed flap length to be adequate. While a defect in the diaphragma sellae and CSF leak were identified in 21 patients during surgery, postoperative CSF leak occurred in only one patient.
Conclusions:The posterior nasoseptal flap provides adequate coverage of the surgical defect and is nearly always successful in preventing postoperative CSF leak following endoscopic transsphenoidal resection of pituitary adenomas. The flap is raised from mucoperiosteum lining the posterior nasal septum, which is otherwise resected during posterior septectomy. Because the anterior septal cartilage is not denuded, raising such flaps avoids the postoperative morbidity associated with the larger Hadad-Bassagasteguy nasoseptal flap.
Keywords: Cerebrospinal fluid leak, closure, endoscopy, nasoseptal flap, pituitary adenoma
INTRODUCTION
Pituitary adenomas are a common benign intracranial neoplasm comprising 15.5% of all primary central nervous system (CNS) neoplasms.[
Transsphenoidal pituitary surgery may be performed either microscopically or endoscopically. While both techniques are cost-effective compared to medical therapies alone in patients with a life expectancy greater than ten years,[
Repairing CSF leaks is crucial in preventing serious postoperative infectious complications. The creation of a fistula between the subarachnoid space and nasal cavity carries the risks of bacterial spread from the nasal cavity, potentially leading to ascending meningitis.[
Nasoseptal and middle turbinate rotational flaps have been used since the 1950s, but these early flaps had a random blood supply and unfavorable arcs of rotation. Hadad and Bassagasteguy developed an improved, pedicled vascularized nasoseptal flap in 2006.[
MATERIALS AND METHODS
This study represents a retrospective analysis of a prospectively constructed database of 43 serial patients who underwent endoscopic transsphenoidal resection of pituitary adenomas with posterior nasoseptal flap placement at our institution between March, 2014 and June, 2015. Patient charts were reviewed for a number of preoperative, intraoperative, and postoperative parameters, including intra and postoperative CSF leaks. This database does not include patients who were deemed to require an extended transsphenoidal approach for resection of their tumor during preoperative surgical planning. Such patients were reconstructed with Hadad-Bassagasteguy nasoseptal flaps.
Surgical technique
After the induction of general endotracheal anesthesia, the patient's nose is decongested with oxymetazoline-soaked cottonoids. The nasal passages are carefully examined, after which the inferior turbinates are first in-fractured and subsequently out-fractured to improve access via the nasal passages. The middle turbinates are injected with 1% lidocaine with epinephrine (1:100,000) for anesthesia and hemostasis, and lateralized using the Freer elevator. The superior turbinates are also visualized and lateralized, allowing for identification of the sphenoid ostium in the sphenoethmoidal recess bilaterally. For larger tumors or cases in which more lateral access is needed, the middle and/or superior turbinates may be partially resected.
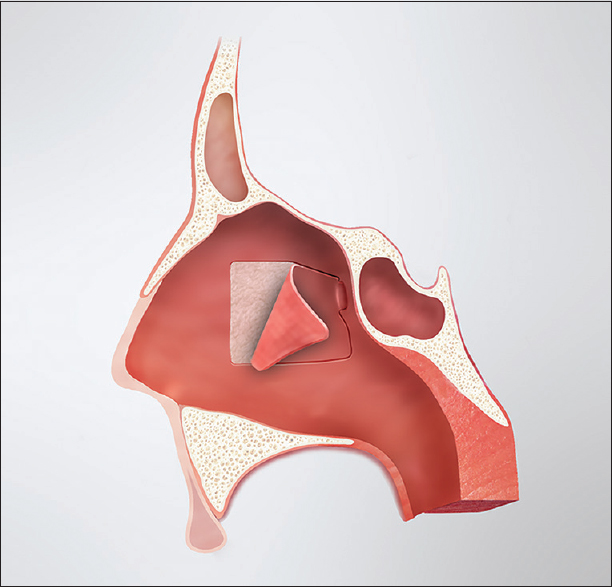
The posterior nasoseptal flap is typically raised on the left side unless anatomic or surgical considerations dictate otherwise; however, this is just a matter of surgeon preference. The anterior aspect of the middle turbinate marks the anterior extent of the posterior nasoseptal flap. Directly medial to this point, the mucosa of the midnasal septum is cauterized vertically and incised. From there, the mucoperiosteum is raised posteriorly to the level of the sphenoid rostrum. The sphenoid ostium is identified submucosally as an anatomic landmark, and the septal mucoperiosteum is transected inferiorly along the maxillary crest and dorsally below the expected level of the olfactory epithelium. This creates a posterior septal flap, pedicled on the posterior septal branch of the sphenopalatine artery [
The mucosa of the contralateral septum is then similarly cauterized and incised. The flap is raised from that point posteriorly and the ostium of the sphenoid is identified. The mucosa and bone of the posterior septum on this second side is then resected, thus creating a central surgical corridor between the turbinates with access to both sphenoid sinuses. Because a posterior septectomy is performed to facilitate a binostril approach for tumor resection, the posterior septal flap can be preserved on both sides if it is felt to be necessary for reconstruction.
After elevation of the flap(s) and creation of a central surgical corridor with access to both sphenoid sinuses, the standard tumor resection is performed by the neurosurgeon. If an intraoperative CSF leak occurs, the dural opening is repaired using medium implantable (0.5–1.0 mm) AlloDerm (LifeCell Corporation, New Jersey, USA) placed in an “inlay” fashion in the epidural space just deep to the residual bone of the sella. The graft is then covered with a thin layer of Tisseel (Baxter Inc., Illinois, USA) tissue adhesive to maintain its position.
Whether there has been an intraoperative CSF leak, the posterior septal flap is rotated into position covering the demucosalized bone of the sphenoid rostrum and floor as well as the area of the dural opening. Intersinus septations are removed and the sphenoid rostrum is drilled as needed to allow for direct overlay of the flap along the floor of the sella. The flap is covered with Tisseel tissue adhesive and Gelfoam. A sphenoid pack consisting of 0.5-inch Nu Gauze (Johnson and Johnson, New Jersey, USA) coated in bacitracin ointment is then placed to maintain the position of the flap and underlying grafts. The pack is brought out through the nasal cavity and secured to the membranous septum with a single suture. At the completion of the procedure, the middle turbinates are medialized to their normal anatomic position.
Postoperative management
Patients are monitored postoperatively for signs of CSF leak, diabetes insipidus, and pituitary dysfunction, and given stool softeners to reduce straining. For patients who did not have intraoperative CSF leaks, the nasal packing is removed before discharge (typically postoperative day 2 or 3). If an intraoperative CSF leak did occur, the packing is left in place until the 1-week postoperative visit.
Calculation of tumor volume
Tumor volumes were calculated based on the (A × B × C)/2 formula, where A, B, and C represent the largest tumor dimension in the anterior-posterior, superior-inferior, and medio-lateral dimensions.
Statistics
All statistical analyses were performed with Prism (Graphpad). Population means were compared with Student's t-test. Population statistics are presented as mean ± standard error. Linear correlations were analyzed with Spearman correlation coefficient. Significance was set at P < 0.05.
RESULTS
Patient information
Patient demographic information and data related to tumors and surgical parameters are shown in
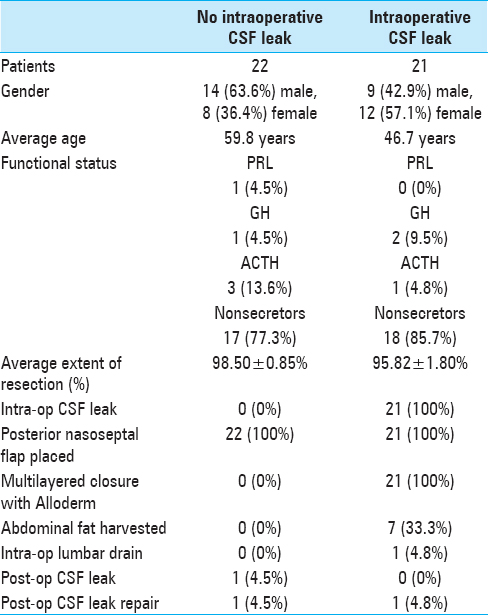
Intraoperative CSF leaks were noted in 21 (48.8%) of the patients [
Postoperatively, 2 patients were treated for presumed CSF rhinorrhea, but a CSF leak was confirmed in only one of them. This patient had no documented intraoperative leak, and the skull base defect had been repaired with the posterior septal flap. However, postoperatively, the patient experienced difficulty in breathing and oxygen desaturation, which led us to remove the nasal packing. The leak occurred subsequent to the packing removal while the patient was straining during a bowel movement. She was treated with secondary surgical repair and lumbar drain placement. The second patient had a small intraoperative leak repaired with the multilayered closure. Clear rhinorrhea, suspicious for a CSF leak, was noted on postoperative day 1. A lumbar drain was placed before β2-transferrin results returned negative, indicating that there was, in fact, no CSF leak. Overall, the posterior nasoseptal flap has a 97.7% success rate in preventing postoperative CSF leaks despite a 48.8% rate of observable intraoperative leaks. Furthermore, barring extenuating circumstances, such as Valsalva maneuvers, the success rate is expected to be even closer to 100%.
Planning the posterior nasoseptal flap
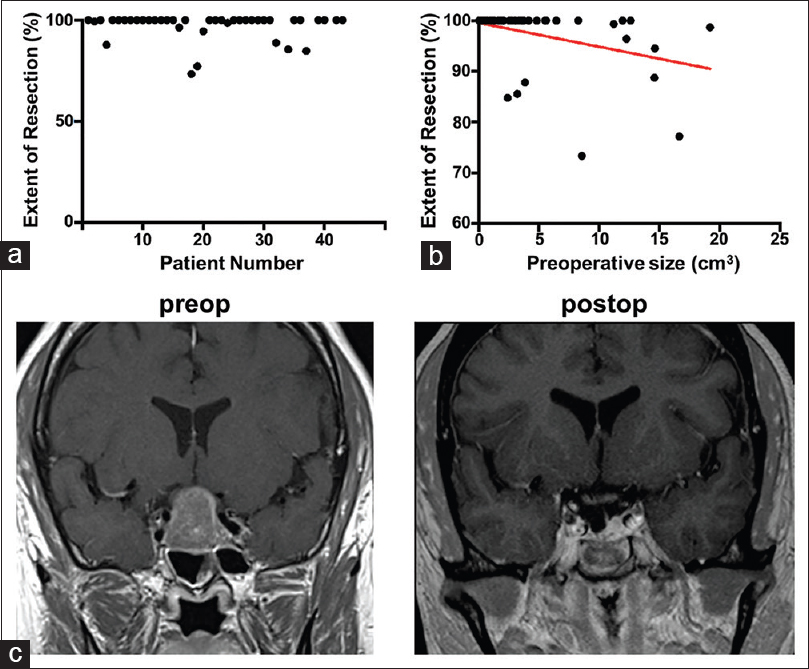
A review of the preoperative MRI showed the distance between the sphenoid rostrum and the middle turbinate (the length of the flap) to be longer than the distance between the sphenoid rostrum and the posterior sphenoid (the length of the defect) in all but 4 cases; on average, it was 5.61 ± 0.69 mm longer [
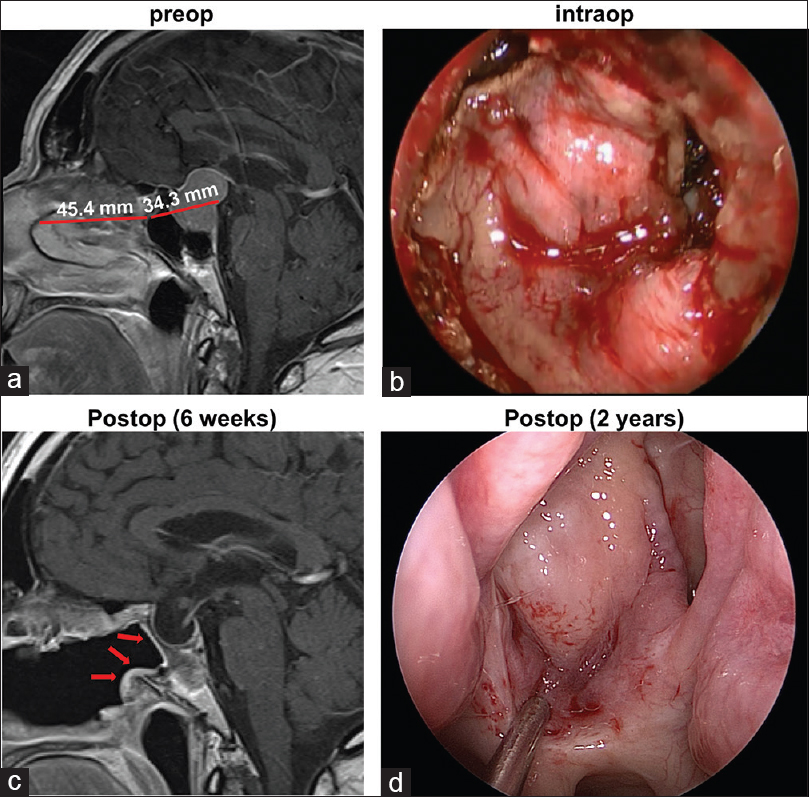
Figure 3
(a) Representative preoperative sagittal gadolinium-enhanced T1-weighted image demonstrates the lengths of the flap and skull defect. (b) Intraoperative photograph through the endoscope demonstrates the posterior nasoseptal flap covering the skull defect. (c) In the postoperative MRI, the vascularized flap (arrows) can be visualized lining the patent sphenoid sinus. (d) Endoscopic photograph of the flap 2 years after the initial surgery
DISCUSSION
Cerebrospinal fluid leak repair
The pituitary gland, seated in the sella turcica, is considered an intradural, extra-arachnoidal part of the brain by virtue of the diaphragma sellae, a reflection of the arachnoid, that separates it from the suprasellar cistern. Because the pituitary is extra-arachnoidal, CSF leaks do not occur as a matter of course during pituitary surgery but rather as a consequence of violation of the diaphragma sellae during tumor resection. The attempt to achieve gross total tumor resection often results in violation of the diaphragma sellae. Our intraoperative CSF leak rate of approximately 49% matches previously published reports,[
Persistent CSF leaks after pituitary adenoma surgery are a concern because they can lead to ascending meningitis. Multiple repair techniques have been described over the years. In 2006, Hadad and Bassagasteguy described a pedicled nasoseptal flap with excellent vascular supply and a wide arc of rotation, and reported success rates in excess of 90%.[
The Hadad-Bassagestaguy flap, though extremely effective, was conceived for use in the setting of intraoperative CSF leaks in extended transsphenoidal approaches for resection of skull base tumors other than pituitary adenomas.[
The posterior nasoseptal flap represents a modification of the Hadad-Bassagestaguy flap, but is smaller and consists primarily of mucosal tissue that is otherwise discarded during the posterior septectomy for the binostril transsphenoidal approach. As such, the crusting associated with denuded septal cartilage after raising Hadad-Bassagestaguy flaps is minimized with posterior flaps. Another fundamental difference between the two approaches is that we raise the posterior flap in all surgeries, regardless of anticipated or observed intraoperative CSF leak, without much added operative time.
In our experience, the posterior septal flap provides excellent coverage for surgical defects associated with endoscopic transsphenoidal surgery for pituitary adenomas, obviating the need for a full-length nasoseptal flap. The posterior nasoseptal flap has the additional advantage of being placed in all cases, thus preventing postoperative leaks from arachnoidal defects and small CSF leaks that may not have been noted during the procedure, as occurred in one of the patients in the study. Importantly, using this flap in all surgical cases for endoscopic pituitary adenectomy virtually eliminates the risk of postoperative CSF leak. This allows us to pursue aggressive resection of pituitary adenomas, as evidenced by the 97% extent of resection in our series. It should be emphasized that this flap is not conceived for use in cases involving larger skull base defects, as in extended transsphenoidal or transnasal approaches, which require the use of full Hadad-Bassagestaguy flaps.
Multilayered closure in cases with documented intraoperative cerebrospinal fluid leak
In cases where we identify a CSF leak intraoperatively, we employ a multilayered approach for closure of the skull defect. A layer of Alloderm is placed in the epidural space tucked under the edges of the remaining sellar bone and covering the dural opening at the floor of the sella. After a thin layer of Tisseel, we place the posterior nasoseptal flap over the Alloderm. This strategy provides two barriers preventing postoperative leaks. We only rarely have to consider use of abdominal fat graft or lumbar drain using this approach. In fact, none of our patients with documented intraoperative CSF leaks had postoperative CSF rhinorrhea. The only patient in our cohort with postoperative rhinorrhea developed it as a result of straining despite no evidence of an intraoperative leak.
Alloderm has been previously used by itself for closure of skull base defects in endoscopic surgery.[
Crusting
The Hadad-Bassagasteguy nasoseptal flap causes donor site morbidity because of exposure of the anterior septal cartilage. This denuded cartilage takes months to fully re-mucosalize and requires repeated office debridement, as well as the use of topical ointments and saline rinses during the healing period.[
Sphenoid sinusitis
In a study of 200 patients who underwent microscopic transsphenoidal hypophysectomy between 1998 and 2001, the incidence of isolated sphenoid sinusitis in the year following surgery was 7.5%.[
CONCLUSIONS
The posterior nasoseptal flap is a novel technique that uses septal mucoperiosteum, which would otherwise be resected during the posterior septectomy, to prevent postoperative CSF leaks following endoscopic transsphenoidal resection of pituitary adenomas. The flap is raised in all cases, regardless of anticipated or observed intraoperative CSF leak. In addition, the flap provides early vascularized coverage of the demucosalized bone of the sphenoid sinus. It is raised at the beginning of the case and provides adequate coverage of the sellar defect without the need for further extension of the flap in the event of an intraoperative CSF leak. In our experience, the flap heals well, maintains a patent sphenoidotomy, and can be elevated and reused if revision surgery is necessary. This approach is uncomplicated, safe, and has advantages relative to alternative flap techniques, such as the rescue flap and the Hadad-Bassagasteguy nasoseptal flap. While not appropriate for closure of larger approaches, the use of the posterior nasoseptal flap virtually eliminates the risk of postoperative CSF leak after pituitary adenectomy, thereby allowing surgeons to pursue complete resection of these benign tumors despite the elevated rate of intraoperative CSF leak associated with aggressive surgery. For these reasons, we propose that the posterior nasoseptal flap is an excellent option for skull defect closure following endoscopic transsphenoidal pituitary adenectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aghi MK, Chen CC, Fleseriu M, Newman SA, Lucas JW, Kuo JS. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Management of Patients With Nonfunctioning Pituitary Adenomas: Executive Summary. Neurosurgery. 2016. 79: 521-3
2. Barkhoudarian G, Cutler AR, Yost S, Lobo B, Eisenberg A, Kelly DF. Impact of selective pituitary gland incision or resection on hormonal function after adenoma or cyst resection. Pituitary. 2015. 18: 868-75
3. Batra P, Citardi M, Lanza D. Isolated sphenoid sinusitis after transsphenoidal hypophysectomy. Am J Rhinol. 2005. 19: 185-9
4. Beltrame S, Toscano M, Goldschmidt E, Garategui L, Campero A, Yampolsky C. Endoscopic treatment of 140 pituitary tumors, results and complications. Neurocirugia (Astur). 2016. p.
5. Bernal-Sprekelsen M, Bleda-Vazquez C, Carrau RL. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 2000. 14: 257-59
6. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997. 40: 225-
7. Dallapiazza R, Grober Y, Starke R, Laws E, Jane J. Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. 2015. 76: 42-52
8. Daudia A, Biswas D, Jones N. Risk of meningitis with cerebrospinal fluid rhinorrhea. Ann Otol Rhinol Laryngol. 2007. 116: 902-5
9. de Almeida JR, Snyderman CH, Gardner PA, Carrau RL, Vescan AD. Nasal morbidity following endoscopic skull base surgery: A prospective cohort study. Head Neck. 2011. 33: 547-51
10. de Divitiis E, Laws ER, Giani U, Iuliano SL, de Divitiis O, Apuzzo ML. The current status of endoscopy in transsphenoidal surgery: An international survey. World Neurosurg. 2015. 83: 447-54
11. de Paiva Neto MA, Vandergrift A, Fatemi N, Gorgulho AA, Desalles AA, Cohan P. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol (Oxf). 2010. 72: 512-9
12. Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH. Cavernous Sinus Invasion in Pituitary Adenomas: Systematic Review and Pooled Data Meta-analysis of Radiological Criteria and Comparison of Endoscopic and Microscopic Surgery. World Neurosurg. 2016. 96: 36-46
13. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML. The prevalence of pituitary adenomas. Cancer. 2004. 101: 613-9
14. Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008. 63: 709-19
15. Fujimoto Y, Balsalobre L, Santos FP, Vellutini E, Stamm AC. Endoscopic combined “transseptal/transnasal” approach for pituitary adenoma: Reconstruction of skull base using pedicled nasoseptal flap in 91 consecutive cases. Arq Neuropsiquiatr. 2015. 73: 611-5
16. Gao Y, Zheng H, Xu S, Zheng Y, Wang Y, Jiang J. Endoscopic Versus Microscopic Approach in Pituitary Surgery. J Craniofac Surg. 2016. 27: e157-9
17. Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: A meta-analysis. World J Surg Oncol. 2014. 12: 94-
18. Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: Efficacy in a large case series. World Neurosurg. 2013. 80: 563-8
19. Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: A systematic review and meta-analysis. Clin Otolaryngol. 2011. 36: 212-20
20. Guvenc G, Kizmazoglu C, Pinar E, Imre A, Kaya I, Bezircioglu H. Outcomes and Complications of Endoscopic Versus Microscopic Transsphenoidal Surgery in Pituitary Adenoma. J Craniofac Surg. 2016. 27: 1015-20
21. Hadad G, Bassagasteguy L, Carrau R, Mataza J, Kassam A, Snyderman C. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006. 116: 1882-6
22. Horridge M, Jesurasa A, Olubajo F, Mirza S, Sinha S. The use of the nasoseptal flap to reduce the rate of post-operative cerebrospinal fluid leaks following endoscopic trans-sphenoidal surgery for pituitary disease. Br J Neurosurg. 2013. 27: 739-41
23. Hu F, Gu Y, Zhang X, Xie T, Yu Y, Sun C. Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World Neurosurg. 2015. 83: 181-7
24. Ivan M, Iorgulescu J, El-Sayed I, McDermott M, Parsa A, Pletcher S. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015. 22: 48-54
25. Jakimovski D, Bonci G, Attia M, Shao H, Hofstetter C, Tsiouris AJ. Incidence and significance of intraoperative cerebrospinal fluid leak in endoscopic pituitary surgery using intrathecal fluorescein. World Neurosurg. 2014. 82: e513-23
26. Jethwa P, Patel T, Hajart A, Eloy J, Couldwell W, Liu J. Cost-Effectiveness Analysis of Microscopic and Endoscopic Transsphenoidal Surgery Versus Medical Therapy in the Management of Microprolactinoma in the United States. World Neurosurg. 2016. 87: 65-76
27. Kassam A, Carrau R, Snyderman C, Thomas A, Vescan A, Prevedello D. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008. 63: ONS44-53
28. Kimple A, Leight W, Wheless S, Zanation A. Reducing nasal morbidity after skull base reconstruction with the nasoseptal flap: Free middle turbinate mucosal grafts. Laryngoscope. 2012. 122: 1920-4
29. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012. 15: 150-9
30. Kuo JS, Barkhoudarian G, Farrell CJ, Bodach ME, Tumialan LM, Oyesiku NM. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Surgical Techniques and Technologies for the Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016. 79: E536-8
31. Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: A prospective cohort study. J Neurosurg. 2015. 123: 799-807
32. Lorenz RR, Dean RL, Hurley DB, Chuang J, Citardi MJ. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope. 2003. 113: 496-501
33. Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Primary Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016. 79: E533-5
34. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World Neurosurg. 2016. 89: 442-53
35. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World neurosurgery. 2016. 89: 442-53
36. Mascarenhas L, Moshel YA, Bayad F, Szentirmai O, Salek AA, Leng LZ. The transplanum transtuberculum approaches for suprasellar and sellar-suprasellar lesions: Avoidance of cerebrospinal fluid leak and lessons learned. World Neurosurg. 2014. 82: 186-95
37. McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. 2015. 123: 813-20
38. McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. 2013. 118: 613-20
39. Mehta G, Oldfield E. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas: Clinical article. J Neurosurg. 2012. 116: 1299-303
40. Neligan P, Mulholland S, Irish J, Gullane P, Boyd J, Gentili F. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996. 98: 1159-68
41. Ostrom Q, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-oncology. 2013. 15: ii1-ii56
42. Paluzzi A, Fernandez-Miranda J, Stefko S, Challinor S, Snyderman C, Gardner P. Endoscopic endonasal approach for pituitary adenomas: A series of 555 patients. Pituitary. 2014. 17: 307-19
43. Rivera-Serrano C, Snyderman C, Gardner P, Prevedello D, Wheless S, Kassam A. Nasoseptal “rescue” flap: A novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011. 121: 990-3
44. Schaberg MR, Anand VK, Schwartz TH, Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg. 2010. 18: 8-14
45. Schlosser R, Bolger W. Nasal cerebrospinal fluid leaks: Critical review and surgical considerations. Laryngoscope. 2004. 114: 255-65
46. Schmidt RF, Choudhry OJ, Takkellapati R, Eloy JA, Couldwell WT, Liu JK. Hermann Schloffer and the origin of transsphenoidal pituitary surgery. Neurosurg Focus. 2012. 33: E5-
47. Seiler R, Mariani L. Sellar reconstruction with resorbable Vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: A 10-year experience with 376 patients. J Neurosurg. 2000. 93: 762-5
48. Shin S, Tormenti M, Paluzzi A, Rothfus W, Chang Y, Zainah H. Endoscopic endonasal approach for growth hormone secreting pituitary adenomas: Outcomes in 53 patients using 2010 consensus criteria for remission. Pituitary. 2013. 16: 435-44
49. Singh H, Essayed W, Cohen-Gadol A, Zada G, Schwartz T. Resection of pituitary tumors: Endoscopic versus microscopic. J Neurooncol. 2016. p.
50. Singh H, Essayed WI, Cohen-Gadol A, Zada G, Schwartz TH. Resection of pituitary tumors: Endoscopic versus microscopic. J Neurooncol. 2016. 130: 309-17
51. Strychowsky J, Nayan S, Reddy K, Farrokhyar F, Sommer D. Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: Systematic review. J Otolaryngol Head Neck Surg. 2011. 40: 175-
52. Tabaee A, Anand V, Barrón Y, Hiltzik D, Brown S, Kacker A. Endoscopic pituitary surgery: A systematic review and meta-analysis. J Neurosurg. 2009. 111: 545-54
53. Tabaee A, Placantonakis DG, Schwartz TH, Anand VK. Intrathecal fluorescein in endoscopic skull base surgery. Otolaryngol Head Neck Surg. 2007. 137: 316-20
54. Thorp B, Sreenath S, Ebert C, Zanation A. Endoscopic skull base reconstruction: A review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurgery. 2014. 37: E4-
55. Wang F, Zhou T, Wei S, Meng X, Zhang , J , Hou Y. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc. 2015. 29: 1270-80
56. Xu T, Peng L, Li H, Wang Y, Liu L, Jiang Y. The safety and efficacy of endoscopic versus microscopic surgery for transsphenoidal pituitary adenoma in China: An updated and cumulative meta-analysis. Zhonghua Yi Xue Za Zhi. 2015. 95: 3378-81