- Division of Neurosurgery, Department of Surgery, University of Saskatchewan, Saskatchewan, Canada
- Division of Neurosurgery, Department of Surgery, University of British Columbia, British Columbia, Canada
- Department of Pathology, University of Alberta, Alberta, Canada
- Division of Neurosurgery, Department of Surgery, University of Alberta, Alberta, Canada
Correspondence Address:
M. M. Chow
Division of Neurosurgery, Department of Surgery, University of Alberta, Alberta, Canada
DOI:10.4103/sni.sni_310_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: A. R. Persad, Y. H. Khormi, F. van Landeghem, M. M. Chow. Unusual case of hemangioblastoma of the cerebellopontine angle. 01-Nov-2017;8:264
How to cite this URL: A. R. Persad, Y. H. Khormi, F. van Landeghem, M. M. Chow. Unusual case of hemangioblastoma of the cerebellopontine angle. 01-Nov-2017;8:264. Available from: http://surgicalneurologyint.com/surgicalint-articles/unusual-case-of-hemangioblastoma-of-the-cerebellopontine-angle/
Abstract
Background:Hemangioblastomas are the most common primary tumor of the posterior fossa. There are few cases of hemangioblastoma of the cerebellopontine angle (CPA). When present in this location, hemangioblastoma presents a diagnostic challenge as its imaging findings closely resemble those of vestibular schwannoma (VS), which is much more common in the CPA.
Case Description:We report the case of a 42-year-old man presenting with vertigo and diplopia found to have a CPA tumor with imaging resembling VS. He underwent retrosigmoidal resection of his tumor, which was found to be a hemangioblastoma.
Conclusion:Hemangioblastoma, though rare in the CPA, should be considered in the differential diagnosis of CPA tumors.
Keywords: Cerebellopontine angle, CPA, hemangioblastoma
BACKGROUND
Hemangioblastomas are well-differentiated, vascular, benign tumors primarily located in the posterior cranial fossa. They are the most common primary intra-axial tumor located in the posterior fossa in adults comprising ~2.5% of all intracranial tumors.[
CASE DESCRIPTION
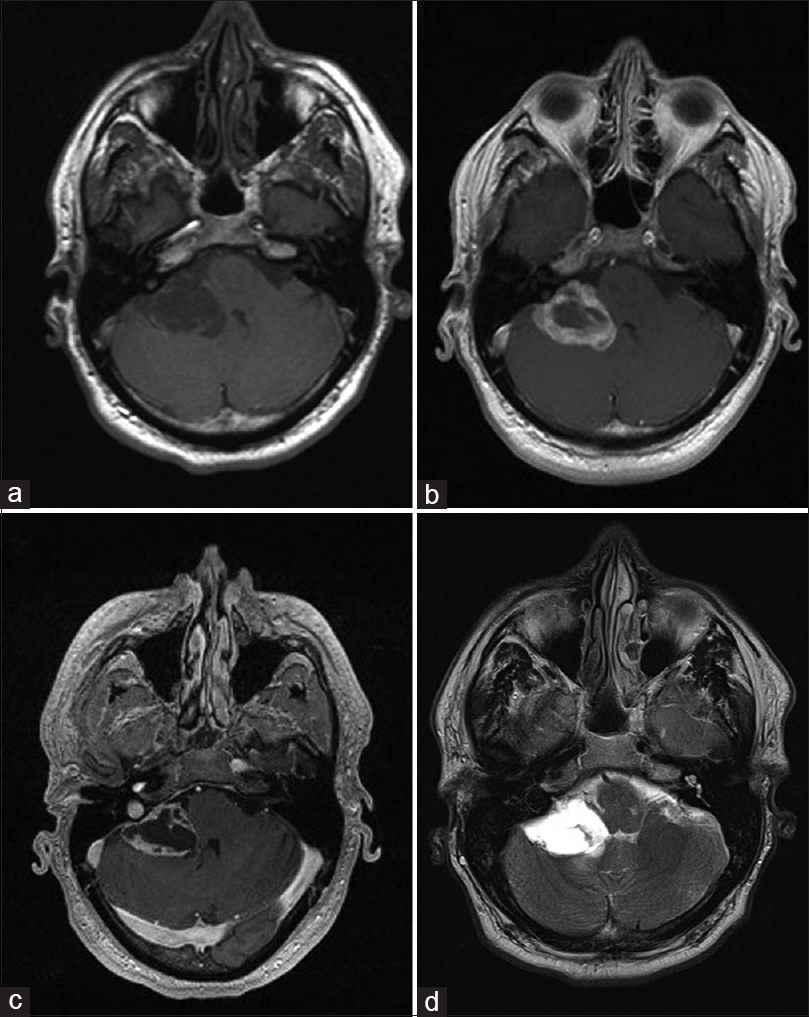
A 42-year-old man presented with vertigo and progressive diplopia on extreme lateral right gaze. He denied tinnitus, hearing loss, headaches, and was found to be grossly neurologically intact. Magnetic resonance (MR) of the brain revealed a lesion demonstrating T1 hypointensity and T2 hyperintensity with peripheral and septal enhancement in the right CPA [
Figure 1
(a) axial T1 weighted, (b) axial FLAIR, (c) axial post-gadolinium and (d) axial T2 weighted MR images of the CP angle tumor. The lesion is hypointense on T1WI, hyperintense on FLAIR and T2WI, and display enhancement along its periphery and septations. Mass effect on the 4th ventricle is appreciated
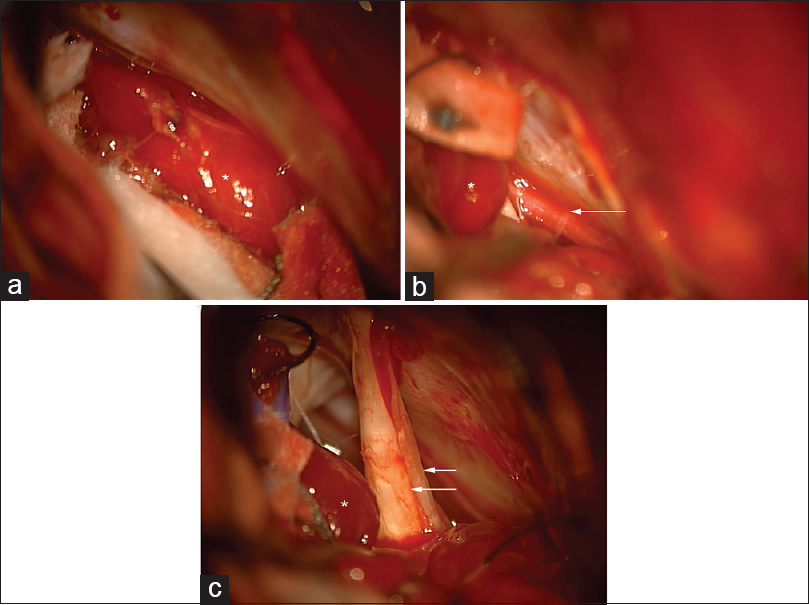
The patient was booked for a retrosigmoid craniotomy for removal of the CPA tumor. A right frontal external ventricular drain (EVD) was placed at the beginning of the case. During craniotomy, retraction of the cerebellum revealed the tumor to be extra-axial [
Figure 2
Intraoperative photographs of the CPA tumor, marked with asterisk (*). (a) Microscopic appearance of the tumor. (b) Microscopic view of the tumor with adjacent anterior inferior cerebellar artery (AICA) (arrow). (c) Microscopic view of CPA tumor with adjacent cranial nerve VII and VIII complex (arrows)
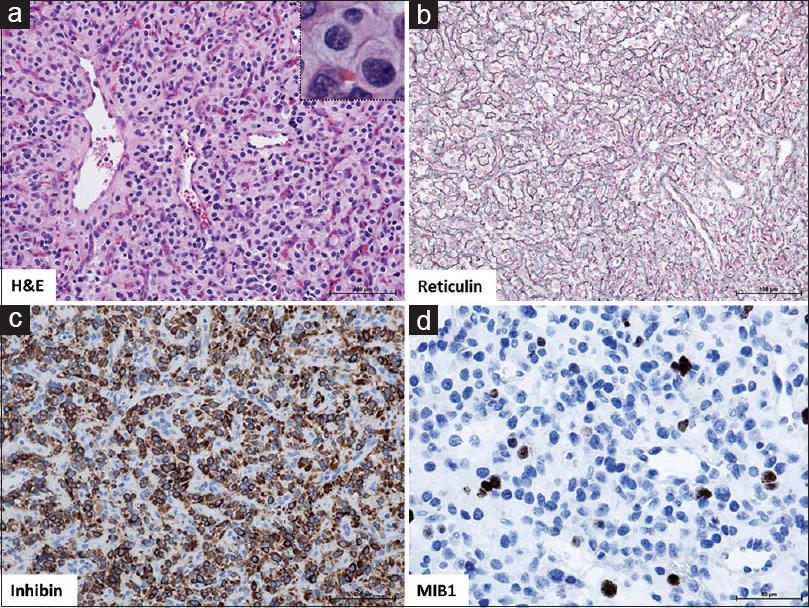
Figure 3
(a) H and E: Tumor composed of epithelioid, slightly foamy stromal cells with mild degree of degenerative atypia and predominant capillary proliferation (reticular variant). No evidence of increased mitotic activity or extramedullary hematopoiesis. ×20 magnification with ×40 inset; (b) Reticulin, highlights reticular fibres of the dense capillary network of this reticular hemangioblastoma, ×20; (c) Inhibin, demonstrates cytoplasmic expression in stromal tumor cells, ×20; (d) MIB1, Proliferation index <5%, ×40
The patient developed a cerebrospinal leak (CSF) leak in his postoperative course, which required re-operation for revision duraplasty. Otherwise his postoperative course was uneventful and he was discharged home with no neurological impairment.
DISCUSSION
Hemangioblastoma is most commonly diagnosed between the third and fifth decades of life and exhibit a male preponderance. They are formed of the remnant mesoderm of the brain. Approximately 25% of cases are associated with von Hippel Lindau (vHL) disease, with 60–84% of vHL patients having hemangioblastoma.[
Typically, hemangioblastomas are found within the cerebellum. Hemangioblastoma involving the CPA is rare.[
Macroscopically, hemangioblastomas can be divided into solid and cystic subtypes. The vast majority, 60–90% of hemangioblastomas are cystic. The typical gross appearance of hemangioblastoma is a reddish or yellow mural nodule with a cystic component. Histologically, hemangioblastoma can de divided into cellular and reticular subtypes. The cellular variant consists of foamy stromal cells clustered in sheets about tangles of capillaries, while in the reticular subtype the stromal cells are evenly distributed around a dense capillary network. Immunohistochemical staining for inhibin, vimentin, and S100 is typically positive in hemangioblastomas.
The differential diagnosis of tumors within the CPA is broad, but the majority of these tumors are VS and meningioma.[
Management of hemangioblastoma is guided by presentation. Serial MRIs can be used to follow asymptomatic lesions. The hypervascularity of hemangioblastoma makes it a surgically challenging lesion. These lesions are typically well circumscribed, and if possible, an en-bloc resection is preferred. Internal debulking of the lesion as in VS resection could lead to disastrous results due to its vascularity. While radiotherapy has been investigated as a treatment,[
CONCLUSION
Hemangioblastoma should be included in the differential diagnosis of CPA tumors. While uncommon, the potential for misdiagnosis and attempt at inappropriate surgical options could be potentially disastrous due to the potential for catastrophic bleeding from these tumors. The retrosigmoidal approach is a safe and effective hearing-preserving approach for resection of these tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amano T, Tokunaga S, Shono T, Mizoguchi M, Matsumoto K, Yoshida F. Cerebellar hemangioblastoma manifesting as hearing disturbance. Neurol Med Chir (Tokyo). 2009. 49: 418-20
2. Bamps S, Calenbergh FV, Vleeschouwer SD, Loon JV, Sciot R, Legius E. What the neurosurgeon should know about hemangioblastoma, both sporadic and in Von Hippel-Lindau disease: A literature review. Surg Neurol Int. 2013. 4: 145-
3. Bonneville F, Sarrazin JL, Marsot-Dupuch K, Iffenecker C, Cordoliani YS, Doyon D. Unusual Lesions of the Cerebellopontine Angle: A Segmental Approach. Radiographics. 2001. 21: 419-38
4. Brackmann DE, Bartels LJ. Rare tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg. 1980. 88: 555-9
5. Bush ML, Pritchett C, Packer M, Ray-Chaudhury A, Jacob A. Hemangioblastoma of the cerebellopontine angle. Arch Otolaryngol Head Neck Surg. 2010. 136: 734-8
6. Cheng J, Liu W, Zhang S, Lei D, Hui X. Clinical features and surgical outcomes in patients with cerebellopontine angle hemangioblastomas: A retrospective series of 23 cases. World Neurosurg. 2017. 103: 248-56
7. Dow GR, Sim DW, O’Sullivan MG. Excision of large solid haemangioblastomas of the cerebellopontine angle by a skull base approach. Br J Neurosurg. 2002. 16: 168-71
8. Friedmann DR, Grobelny B, Golfinos JG, Roland JT. Nonschwannoma tumors of the cerebellopontine angle. Otolaryngol Clin North Am. 2015. 48: 461-75
9. Grahovac G. Solid hemangioblastoma of vestibular nerve mimicking vestibular schwannoma. Neurol Sci. 2015. 36: 1537-9
10. Kamitani H, Hirano N, Takigawa H, Yokota M, Miyata H, Ohama E. Attenuation of vascularity by preoperative radiosurgery facilitates total removal of a hypervascular hemangioblastoma at the cerebellopontine angle: Case report. Surg Neurol. 2004. 62: 238-44
11. Kano H, Niranjan A, Mongia S, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008. 63: 443-51
12. Laviv Y, Thomas A, Kasper EM. Hypervascular Lesions of the Cerebellopontine Angle: The Relevance of Angiography as a Diagnostic and Therapeutic Tool and the Role of Stereotactic Radiosurgery in Management. A Comprehensive Review. World Neurosurg. 2017. 100: 100-17
13. Moffat DA, Ballagh RH. Rare tumours of the cerebellopontine angle. Clin Oncol (R Coll Radiol). 1995. 7: 28-41
14. Moon BH, Park SK, Han YM. Large solid hemangioblastoma in the cerebellopontine angle: Complete resection using the transcondylar fossa approach. Brain Tumor Res Treat. 2014. 2: 128-31
15. Nair BR, Joseph V, Chacko G, Keshava SN. Giant solid hemangioblastoma of the cerebellopontine angle: A technically challenging case. Neurol India. 2014. 62: 228-9
16. Qiao PF, Niu GM, Han XD. Hemangioblastoma originating from the right cerebellopontine angle. Neurosciences (Riyadh). 2011. 16: 372-4
17. Rachinger J, Buslei R, Prell J, Strauss C. Solid haemangioblastomas of the CNS: A review of 17 consecutive cases. Neurosurg Rev. 2009. 32: 37-48
18. Sakamoto N, Ishikawa E, Nakai Y, Akutsu H, Yamamoto T, Nakai K. Preoperative Endovascular Embolization for Hemangioblastoma in the Posterior Fossa. Neurol Med Chir (Tokyo). 2012. 52: 878-84
19. Takeuchi S, Tanaka R, Fujii Y, Abe H, Ito Y. Surgical treatment of hemangioblastomas with presurgical endovascular embolization. Neurol Med Chir (Tokyo). 2001. 41: 246-51
20. Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel- Lindau disease. J Neurosurg. 2003. 98: 82-94
21. Young S, Richardson AE. Solid haemangioblastomas of the posterior fossa: Radiological features and results of surgery. J Neurol Neurosurg Psychiatry. 1987. 50: 155-8