- Departments of Neurosurgery, Lozano Blesa University Clinical Hospital, Av. San Juan Bosco, nº 15, Zaragoza, Spain.
- Departments of Pathology, Lozano Blesa University Clinical Hospital, Av. San Juan Bosco, nº 15, Zaragoza, Spain.
Correspondence Address:
Juan Francisco Sánchez-Ortega
Departments of Neurosurgery, Lozano Blesa University Clinical Hospital, Av. San Juan Bosco, nº 15, Zaragoza, Spain.
DOI:10.25259/SNI_272_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Juan Francisco Sánchez-Ortega, Jesús Aguas-Valiente, Patricia Sota-Ochoa, Juan Calatayud-Pérez. Glioblastoma with primitive neuronal component: A case report and considerations of fluorescence-guided surgery. 04-Jul-2020;11:178
How to cite this URL: Juan Francisco Sánchez-Ortega, Jesús Aguas-Valiente, Patricia Sota-Ochoa, Juan Calatayud-Pérez. Glioblastoma with primitive neuronal component: A case report and considerations of fluorescence-guided surgery. 04-Jul-2020;11:178. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10117
Abstract
Background: Glioblastoma with primitive neuronal components (GB/PNC) is an extremely rare type of glioblastoma characterized by presenting histological and cytogenetic features of both entities. The mixed nature of these tumors limits the imaging diagnosis and supposes a therapeutic dilemma.
Case Description: We present the case of a 77-year-old female with a GB/PNC who is treated with surgery and adjuvant radiochemotherapy according to the STUPP protocol, where an abnormal uptake of 5-aminolevulinic acid (5-ALA) is evident during surgery in probable relation to the mixed nature of GB/PNC.
Conclusion: GB/PNC is extremely rare tumors. Given its low prevalence, there are no studies that refer to the macroscopic characteristics of the tumor as well as evidence of the effectiveness of adjuvant treatment. Fluorescence-guided resection with 5-ALA is the surgical treatment of choice in surgery for high-grade gliomas; however, in GB/PNC, it may not be as useful since PNC may have less fluorescent marker uptake and be more dimly visualized when excited by light using the surgical microscope.
Keywords: 5-aminolevulinic acid, Fluorescence, Glioblastoma, Primitive neuronal components
INTRODUCTION
Glioblastoma with primitive neuronal components (GB/PNC) is a rare and emerging variant of GB, accounting for almost 0.5% cases of GB.[
We present the case of a 77-year-old female patient with a GB/PNC that is treated with surgery and radiochemotherapy according to the STUPP protocol and where an abnormal uptake of 5-aminolevulinic acid (5-ALA) during surgery in probable relationship with the mixed nature of GB/PNC.
CASE REPORT
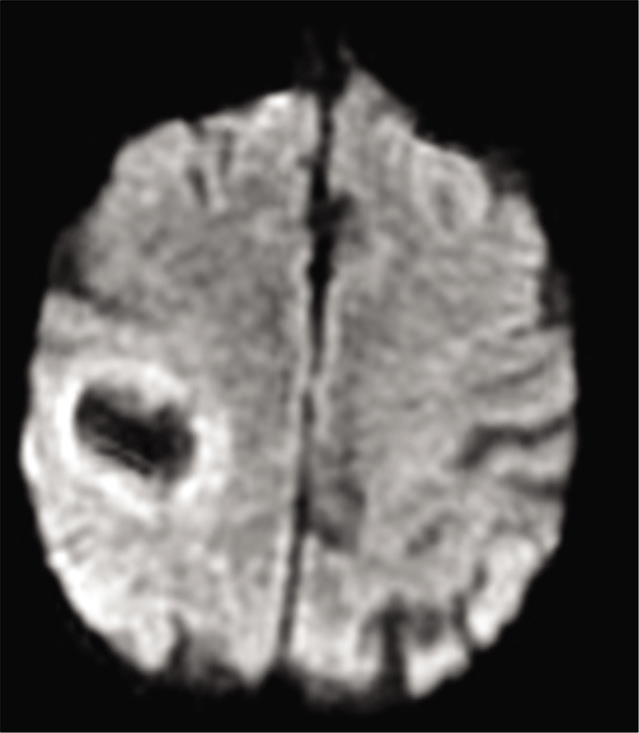
We present the case of a 77-year-old woman with a history of hypertension, dyslipidemia, and atrial fibrillation who presented with hemiparesis and left hemihypoesthesia of 4 months of evolution. Magnetic resonance imaging (MRI) showed an expansive intracranial lesion at the right frontoparietal level with abundant perilesional edema, areas that enhance paramagnetic contrast and a marked restriction of diffusion in the peripheral region [
Figure 4:
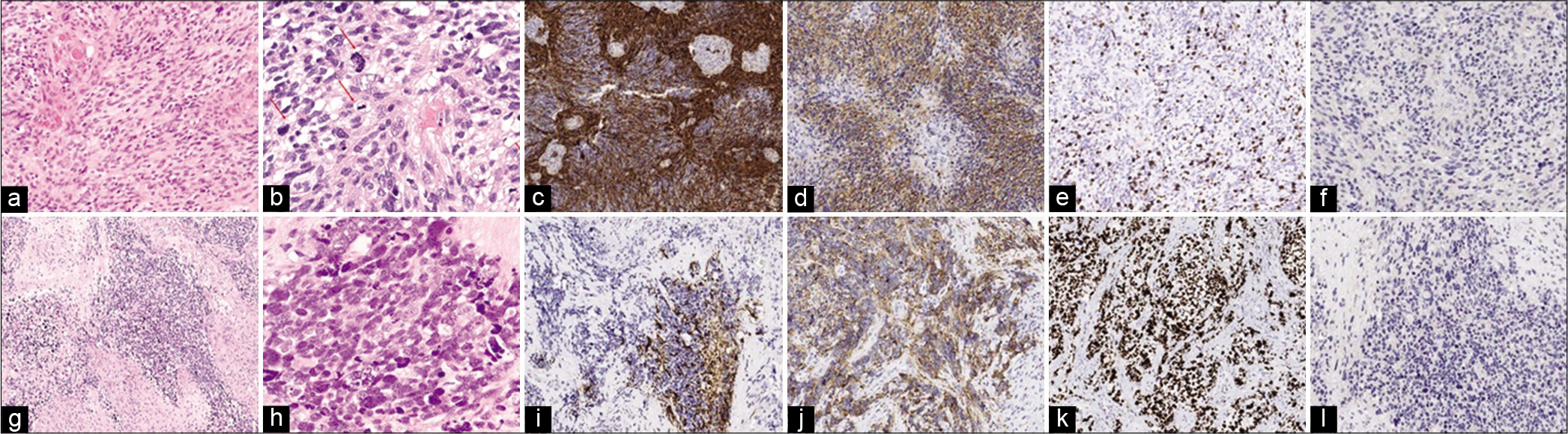
(a) Hematoxylin-eosin stain showing a tumor with dense cellularity and glomeruloid vessels GB-compatible (×10). (b) Hematoxylin- eosin stain showing atypical cellularity with marked nuclear pleomorphism and abundant mitosis (×20). (c) Gliofibrillar acid protein (GFAP) staining showing glial line hypercellularity (×10). (d) Synaptophysin staining showing negative immunoreactivity (×10). (e) MIB-1 staining showing the proliferation index of glioblastoma (×10). (f) Anti-isocitrate dehydrogenase (IDH)-1 R132H immunohistochemistry showing absence of IDH-1 mutation (IDH wild-type GB) (×10). (g) Hematoxylin-eosin stain showing solid-looking primitive nodules presenting neuronal differentiation separated by a desmoplasic stroma (×10). (h) Hematoxylin-eosin stain showing high nuclear-cytoplasmic ratio, karyorrhexis, and occasional cell wrapping (×20). (i) GFAP staining showing low GFAP expression within the foci of the primitive neuronal component in contrast to the glioma component (×10). (j) Synaptophysin staining showing positive immunoreactivity in primitive cells (×10). (k) MIB-1 staining showing a high Ki-67 labeling index with nuclear positivity for more than 90% of tumor cells PNC-compatible (×10). (l) Anti-IDH-1 R132H immunohistochemistry showing a negative study for primitive neuronal component (×10).
DISCUSSION
GB/PNC is a rare variant of GB that exhibits very aggressive behavior. As with “conventional” GBs, they are considered Grade IV tumors according to the classification of CNS tumors of the WHO.[
Genetically, GB/PNC can present genetic alterations similar to “gliomas,” such as the 10q and 1p/19q deletions or the amplification of the gene encoding the epidermal growth factor receptor, and characteristics similar to those traditionally called “Primitive neuroectodermal tumors (PNET),” such as the high Ki-67 index or the amplification of the N-myc or C-myc genes. Cases of GB/PNC have been discovered with mutations in the enzyme IDH-1, which supports the origin of these tumors from secondary GB and seems to have a positive prognostic value.[
Regarding imaging, although the MRI findings do not allow a distinction to be made between a “conventional” GB and a GB/PNC; the reduction of the apparent diffusion coefficient and the restriction in diffusion may be more evident probably due to the hypercellularity of the PNC.[
The treatment of these tumors is based on surgery, radiotherapy, and chemotherapy. Surgery plays a primary role in the treatment of high-grade gliomas and has been associated with increased survival in those patients treated with complete resection and adjuvant radiochemotherapy compared to conservatively treated patients.[
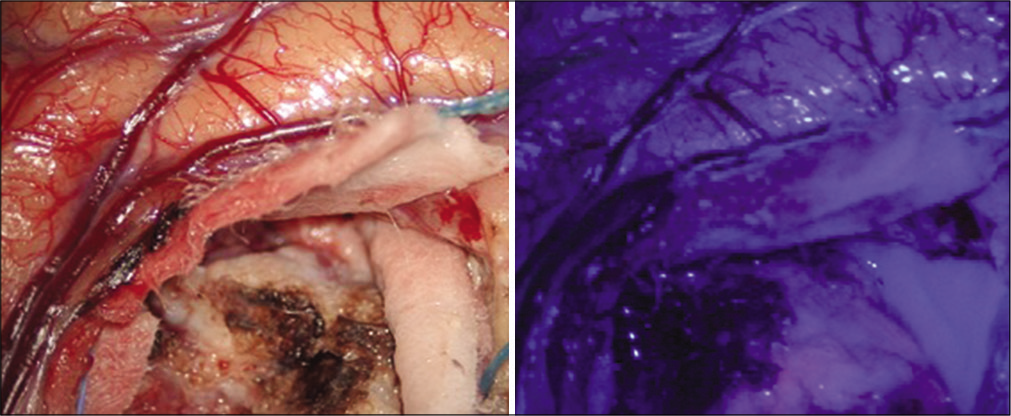
Guided fluorescence with 5-ALA facilitates surgical resection of high-grade gliomas since it allows to differentiate tumor tissue from healthy brain parenchyma during surgery. Once administered to the patient, 5-ALA is absorbed by the different tissues of the body and especially by malignant glial cells. When applying a light with a wavelength of 400–410 nm on the tumor tissue using a surgical microscope, protoporphyrin IX emits intense, strong pink fluorescence, allowing the extent of the malignant glioma to be determined and the resection to be guided.
5-ALA is a natural precursor to hemoglobin that is metabolized in the mitochondria, causing fluorescent protoporphyrins, such as protoporphyrin IX. The accumulation of protoporphyrin IX by tumor cells depends on the activity of certain mitochondrial enzymes, such as ferrokelase. High levels of ferrokelase have been shown to promote the accumulation of protoporphyrin IX in tumor cells of glioblastomas, bladder cancer, and lung cancer.[
In relation to CNS tumors, the concentration of protoporphyrin IX is considerably higher in malignant glial cells than in neurons, healthy glial cells, or neoplastic non- glial cells. Marbacher et al.,[
Due to the low prevalence, there is no standard treatment for GB/PNC. Most of the data are derived from small series or case reports. The largest series correspond to Varlet et al.[
CONCLUSION
GB/PNC are extremely rare malignant tumors of the brain. The existence of a mixed tumor progression limits the image diagnosis and supposes a therapeutic dilemma. Fluorescence- guided resection with 5-ALA is the surgical treatment of choice in surgery for high-grade gliomas; however, in GB/PNC, it may not be as useful since PNC may have less fluorescent marker uptake and be more dimly visualized when excited by light using the surgical microscope. To date, there is no standard treatment protocol for GB/PNC and in the reports or case series described in the literature, there is a tendency to recommend a combined therapy for both tumors. In spite of everything, the prognosis remains unfortunate and the contribution of new cases or studies with a greater casuistry can contribute to the understanding of the pathophysiology and establish a targeted therapeutic approach for these malignancies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ali S, Joseph NM, Perry A, Barajas RF, Cha S. Apparent diffusion coefficient in glioblastoma with PNET-like components, a GBM variant. J Neurooncol. 2014. 119: 353-60
2. Borys E, Prabhu VC, Pambuccian S. Glioblastoma with primitive component: Cytologic findings in intraoperative squash preparations. Diagn Cytopathol. 2019. 47: 234-7
3. Ishikawa T, Kajimoto Y, Inoue Y, Ikegami Y, Kuroiwa T. Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv Cancer Res. 2015. 125: 197-216
4. Kimbason T, Turner SG, Kazmi SA, Fourgas E, Gergel T, Belles L. Malignant glioma with primitive neuroectodermal components: Clinical and pathologic features with treatment modalities of five cases. J Clin Exp Pathol. 2015. 5: 255-
5. Laws E, Parney I, Huang W, Anderson F, Morris AM, Asher A. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the glioma outcomes Project. J Neurosurg. 2003. 99: 467-73
6. Louis D, Suva M, Burger P, Perry A, Kleihues P, Aldape KD, Louis D, Ohgaki H, Wiestler O, Cavenee WK.editors. Glioblastoma, IDH-wildtype. WHO Classification of Tumours of the Central Nervous System. France: Lyon IARC Press; 2016. p. 33-4
7. Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014. 36: E10-
8. Omoto K, Matsuda R, Nakai Y, Tatsumi Y, Nakazawa T, Tanaka Y. Expression of peptide transporter 1 has a positive correlation in protoporphyrin IX accumulation induced by 5-aminolevulinic acid with photodynamic detection of non-small cell lung cancer and metastatic brain tumor specimens originating from non-small cell lung cancer. Photodiagnosis Photodyn Ther. 2019. 25: 309-16
9. Perry A, Miller C, Gujrati M, Scheithauer BW, Zambrano SC, Jost SC. Malignant gliomas with primitive neuroectodermal tumor-like components: A clinicopathologic and genetic study of 53 Cases. Brain Pathol. 2009. 19: 81-90
10. Prelaj A, Rebuzzi S, Caffarena G, Berrìos JR, Pecorari S, Fusto C. Therapeutic approach in glioblastoma multiforme with primitive neuroectodermal tumor components: Case report and review of the literature. Oncol Lett. 2018. 15: 6641-7
11. Song X, Andrew R, Terence S, Fung K, Farmer P, Gandhi S. Glioblastoma with PNET-like components has a higher frequency of isocitrate dehydrogenase 1 (IDH1) mutation and likely a better prognosis than primary glioblastoma. Int J Clin Exp Pathol. 2011. 4: 651-60
12. Varlet P, Soni D, Miquel C, Roux F, Meder J, Chneiweiss H. New variants of malignant glioneuronal tumors: A clinicopathological study of 40 Cases. Neurosurgery. 2004. 55: 1377-91