- Department of Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas,
- Department of Radiation Oncology, University of Arkansas for Medical Sciences, Little Rock, Arkansas,
- Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, United States.
Correspondence Address:
John D. Patterson
Department of Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas,
DOI:10.25259/SNI_146_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John D. Patterson, Matthew Helton, Mehdi Khani, Sehrish Sardar, Kevin Thomas, Edvaldo P. Galhardo, Jose A. Penagaricano, John D. Day, Analiz Rodriguez. Neurosurgical management of perineural metastases: A case series and review of the literature. 25-Jul-2020;11:206
How to cite this URL: John D. Patterson, Matthew Helton, Mehdi Khani, Sehrish Sardar, Kevin Thomas, Edvaldo P. Galhardo, Jose A. Penagaricano, John D. Day, Analiz Rodriguez. Neurosurgical management of perineural metastases: A case series and review of the literature. 25-Jul-2020;11:206. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10158
Abstract
Background: Perineural invasion (PNI) and spread are one of the grimmest prognostic factors associated with primary skin and head-and-neck cancers, yet remain an often confused, and underreported, phenomenon. Adding complexity to reaching a diagnosis and treating perineural spread (PNS) is the finding that patients may have no known primary tumor, history of skin cancer, and/or incidental PNI in the primary tumor. These delays in diagnosis and treatment are further compounded by an already slow disease process and often require multidisciplinary care with combinations of stereotactic radiosurgery, surgical resection, and novel treatments such as checkpoint inhibitors.
Methods: Six patients with metastatic cancer to the cranial nerves who underwent Gamma Knife radiosurgery (GKRS) treatment were chosen for retrospective analysis. This information included age, gender, any past surgeries (both stereotactic and regular surgery), dose of radiation and volume of the tumor treated in the GKRS, date of PNS, comorbidities, the patient follow-up, and pre- and post-GKRS imaging. The goal of the follow-up with radiographing imaging was to assess the efficacy of GKSS.
Results: The clinical course of six patients with PNS is presented. Patients followed variable courses with mixed outcomes: two patients remain living, one was lost to follow-up, and three expired with a median survival of 12 months from date of diagnosis. Patients at our institution are ideally followed for life.
Conclusion: Given the morbidity and mortality of PNS of cancer, time is limited, and further understanding is required to improve outcomes. Here, we provide a case series of patients with PNS treated with stereotactic radiosurgery, discuss their clinical courses, and review the known literature.
Keywords: Head-and-neck cancer, Perineural invasion, Perineural spread, Skin cancer, Skull base surgery
INTRODUCTION
Perineural invasion (PNI) and spread are a unique disease manifestation of multiple solid tumors that remain poorly understood across specialties. While head-and-neck squamous cell carcinomas (HNSCCs) are the most common culprit, perineural disease can occur in other solid tumors, such as skin cancers and sinonasal carcinoma. Approximately 95% of head-and-neck cancer cases are diagnosed as HNSCCs, and with a 5-year survival rate below 50%, locoregional failure accounts for the vast majority of deaths. One of the factors implicated in local recurrence of HNSCC is the presence of perineural tumor growth.[
After initial PNI of a primary tumor into adjacent peripheral nerves, tumor cells spread contiguously within the perineural space into cranial nerves. When invasion is extensive enough to cause clinical or radiologic deficits in the involved nerve, it is often referred to as “clinical” PNI or perineural spread (PNS). In comparison, PNI that is only detected on tumor histology in asymptomatic patients is known as “incidental PNI” and is associated with better outcomes.[
Unlike lymphatic and hematogenous metastasis of cancer cells to lymph nodes and solid organs, many patients and clinicians are unfamiliar with this form of cancer spread. It is often confused with other benign causes of cranial nerve dysfunction, such as Bell’s palsy or trigeminal neuralgia (TGN). This contributes to delays in diagnosis, which are further compounded by a slowly progressive disease process. Adding complexity to reaching a diagnosis of PNS is the finding that patients with PNS can have no known primary tumor, history of skin cancer, and/or incidental PNI in the primary tumor.[
METHODS
Six patients with metastatic head-and-neck carcinoma to the cranial nerves that underwent Gamma Knife radiosurgery (GKRS) treatment were chosen for retrospective analysis. The criteria needed to be met by a patient for inclusion were broad given the low incidence and included patients with primary head-and-neck cancer with metastases to the adjacent cranial nerves that had undergone prior GKRS at the University of Arkansas for Medical Sciences (UAMS). For inclusion, patients needed complete records of GKRS, pre- and post-GKRS imaging, survival estimated at >3 months, and pathologic diagnosis confirming primary head-and-neck metastasis. Patients excluded were those without complete records, such as GKRS radiation dose/treatment volume, pathology showing a primary central nervous system (CNS) tumor, presence of leptomeningeal spread at diagnosis, or change to comfort care before completion of treatment. The purpose of GKRS was to ablate the tumor to treat the pain and other associated symptoms of the tumor compressing the nerves and stop further metastases.
On acquiring permission from the Ethical Review Board of UAMS, information on patients meeting the above criteria were obtained from our patient medical record system, primarily EPIC (Epic Systems Corporation, Verona, Wisconsin), to perform a retrospective analysis. This information included age, gender, any past surgeries (both stereotactic and regular surgery), radiation doses and volumes of the tumor treated with GKRS date of PNS, comorbidities, the patient follow-up, and any radiographic imaging. The goal of the follow-up with radiographing imaging was to assess the efficacy of GKRS in treating perineural metastases and was performed at a goal of minimum 3 months post-GKRS.
RESULTS
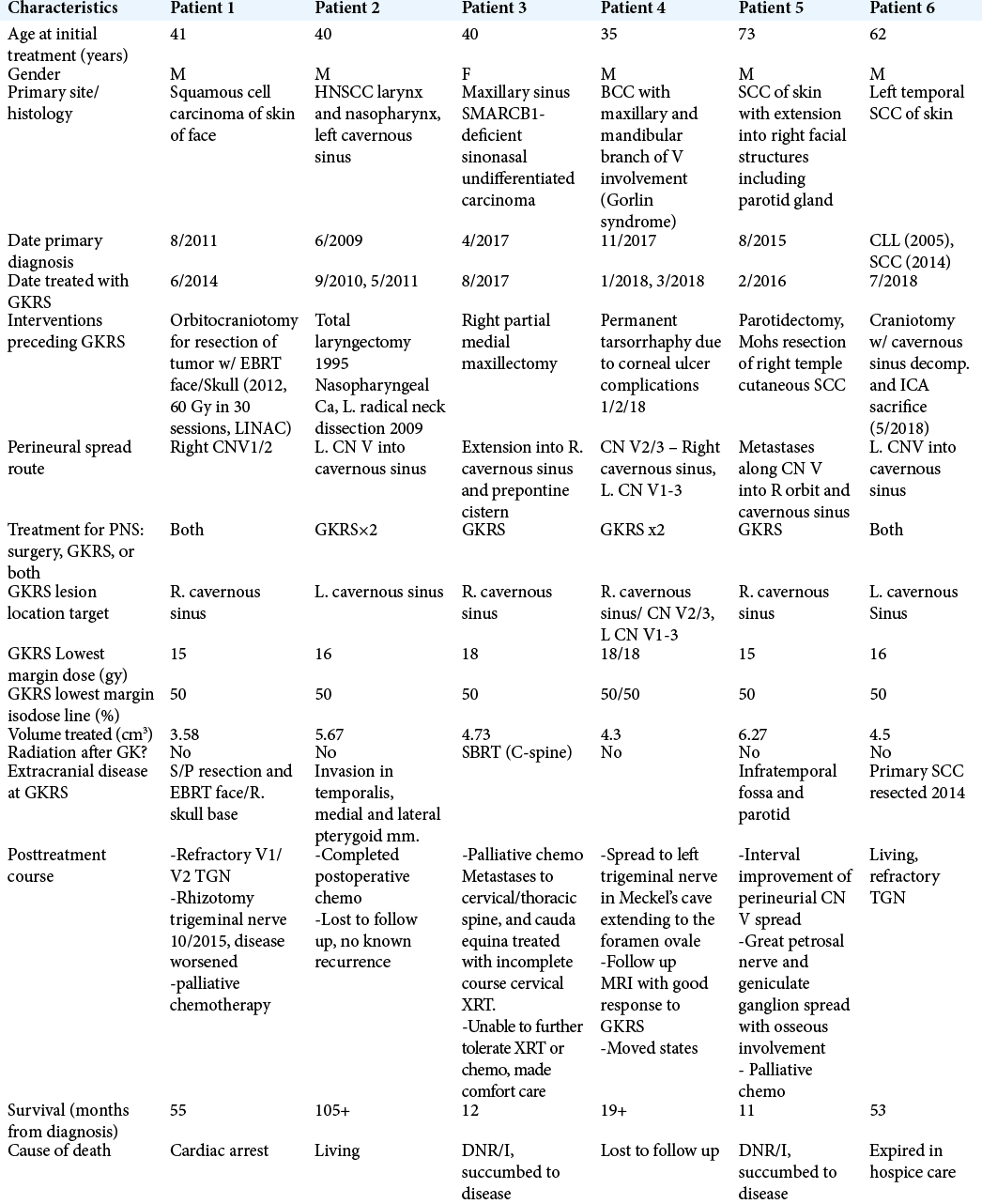
The clinical course of six patients with PNS is presented and followed variable courses with mixed outcomes, detailed characteristics and treatment timeline/dates are provided in [
Patient 1, a 41-year-old male, was diagnosed with primary HNSCC originating from facial skin in 2011 and underwent radical resection and orbitocraniotomy as well as external beam radiotherapy to face and skull. Disease showed evidence of recurrence 2 years later for which the patient underwent GKRS to the right cavernous sinus (6/2014). A rhizotomy was performed 1 year after GKRS for refractory TGN, however, disease shortly worsened and palliative chemotherapy was attempted in late 2015. The patient passed from cardiac arrest in early 2016. Patient 2, a 40-year-old male with primary HNSCC of larynx and nasopharynx, was previously treated with total laryngectomy and radical neck dissection after primary diagnosis in 1995; however, records from 1995 are limited. Disease recurred in 2009 and was treated with a L. radical neck dissection at that time and chemotherapy. One year later, the patient presented after a generalized tonic-clonic seizure and was discovered to have a mass occupying the L. cavernous sinus and temporal lobe, the mass was surgically resected (9/2010) and postoperative GKRS was used to target PNS in the cavernous sinus. GKRS was repeated again in 5/2011, and post-GKRS imaging showed evidence of halted tumor growth, however, the patient was lost to follow up, but remains living. Patient 3, a 41-year-old female, presented in 4/2017 with primary head-and-neck cancer, later confirmed to be SMARCB1-deficient sinonasal carcinoma of the maxillary sinus which followed a course of rapid progression. She shortly underwent R. partial maxillectomy followed by GKRS in 8/2017 for PNS to the cavernous sinus and prepontine cistern. Palliative chemotherapy and SBRT to the cervical spine were performed for spinal metastases, however, the patient succumbed to disease and passed in 5/2018, 1 year after primary disease diagnosis.[ Patient 4, a 35-year-old male with known Gorlin syndrome and presented with basal cell carcinoma (BCC) with PNS of maxillary/mandibular branch of CN V in 11/2017. After undergoing two treatments of GKRS (1/2018 and 3/2018) to perineural lesions in both the right and left cavernous sinus, Magnetic resonance imaging (MRI) results showed tumor regression, however, the patient was lost to follow up after moving to another state. • Patient 5, a 73-year-old male, presented with squamous cell carcinoma (SCC) of the skin in 8/2015 with invasion into parotid gland and facial structures with PNS. The patient was treated with parotidectomy followed by GKRS (2/2016) to the R. cavernous sinus and showed interval improvement, however, progression occurred soon after and the patient succumbed to disease 7/2016. • Patient 6, a 62-year-old male, presented with temporal SCC of skin in 2014 which was treated with local excision. Disease recurred 4 years later with metastasis and PNS and was treated with craniotomy for resection (5/2018) and followed by GKRS (7/2018) to the left cavernous sinus. Patient 6 remained living with refractory TGN and was transitioned to home hospice care where he expired in December 2018.
DISCUSSION
Diagnosis
Our series align with previous studies and show the difficult diagnosis and treatment paradigm that is associated with perineural tumor spread and confirms its high morbidity.[
Two-thirds of our patients [
Due to the unique nature of PNS, the semiology of the disease depends on both the cranial nerves involved, as well as the anatomic location of involvement. While previously known that the trigeminal (V) (most frequent) and facial (VII) nerves are the most commonly involved cranial nerves, location and/or ganglionic involvement are key in presenting symptoms.[
MRI is considered “Gold Standard” modality for the diagnosis of PNS and determination of the extent of tissue involvement. Routine perineural nerve studies include T2 coronal and axial fat-suppressed images, T1 axial/ coronal precontrast, and T1 axial/coronal fat-suppressed postgadolinium MR images.[
For staging and treatment purposes, the disease extent can be classified by a zonal system dictated by the extent of skull base and/or cranial nerve ganglia involvement.[
Extracranial manifestations of disease are further compounded by cranial nerve involvement. Patients with cavernous sinus involvement may present with ophthalmoplegia and masticatory muscle wasting (V3), most prominent at the temporal fossa.[
Treatment
Progression of disease and response to treatment in patients is unpredictable. All of our patients were treated with multidisciplinary approaches including otolaryngology, neurological surgery, radiation and medical oncology, ophthalmology, and dermatology. Complex and aggressive invasion of primary disease requiring multispecialty care, as well as varying symptomatic presentation, is likely the reason for the broad treatment approaches used in each of our six patients, as well as similar series. All patients in our series had prior surgical interventions or procedures by specialties before neurosurgical involvement.
Overall, radiotherapy with or without surgery is the current mainstays of treatment. The extent of progression along a named nerve typically determines whether the patient undergoes resection (including the type of resection), radiotherapy (adjuvant or alone), or is untreatable. There is little consensus among skull base surgeons and radiation oncologists as to the appropriate form of the treatment of PNS. Most reported data are restricted in its clinical interpretation through the use of varying treatment approaches, inconsistent grouping of either incidental PNI and clinical PNI cohorts, or different tumor types.[
In our series, patients 1 and 6 also had surgical adjuvant treatment in addition to radiosurgery. In these cases, the patients continued to have severe refractory pain and underwent decompression of the cavernous sinus to provide symptomatic relief. Entrance into the cavernous sinus during surgery was not conducted as this carries significant risks to critical neurovascular structures.[
Surgical interventions aside, all patients in this series received one or more treatments with GKRS and demonstrated some radiologic improvement or slowed progression of disease. While disease progression or recurrence occurred in some cases following treatment, the use of adjuvant GKRS can provide focused treatment for PNS which follows predicable patterns of spread within the cranial nerves [
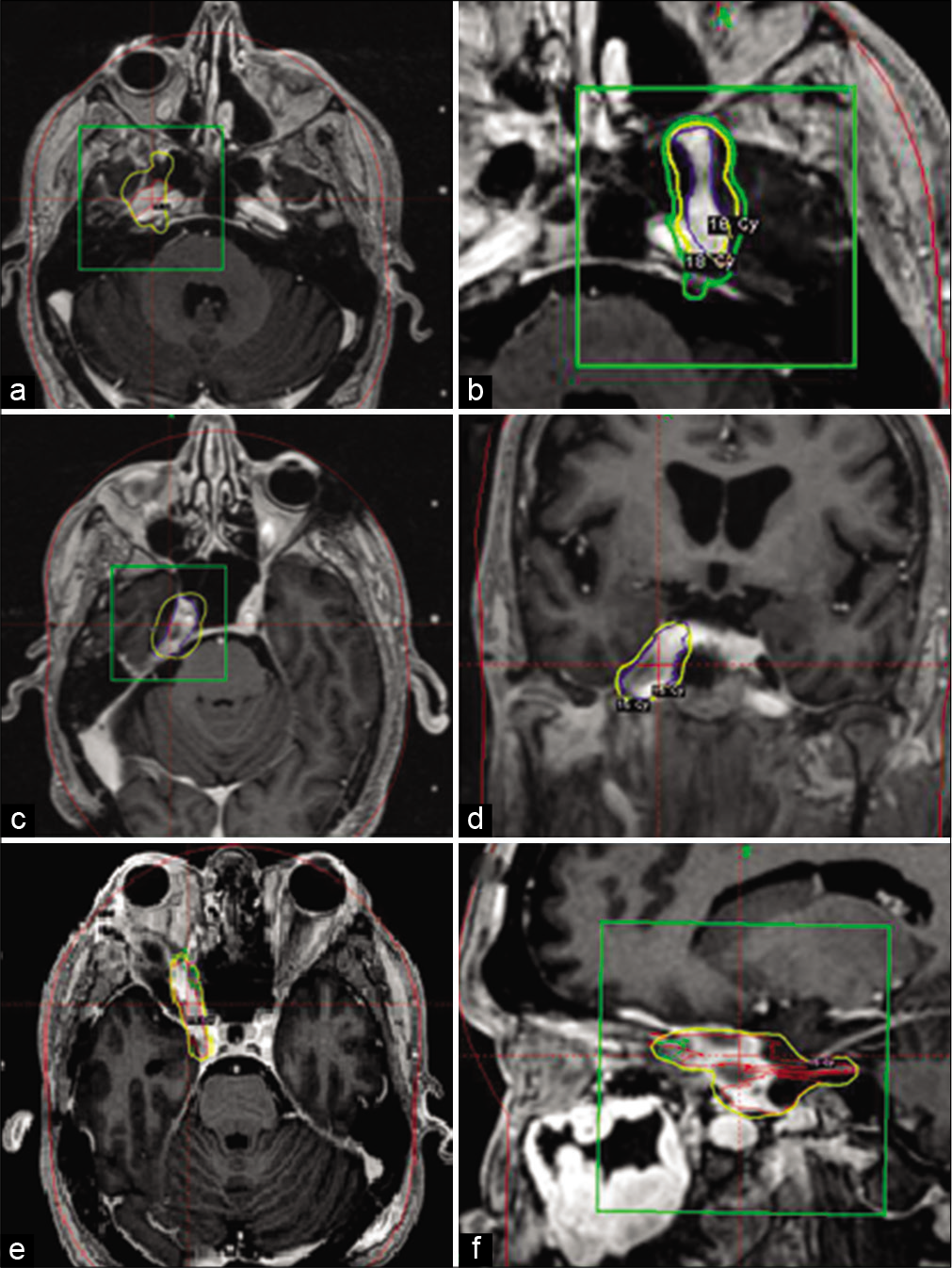
Figure 1:
Magnetic resonance images are presented demonstrating location of Gamma-Knife radiation and perineural extension. a and b, patient 4, axial plane imaging demonstrating cranial nerve V spread in R. and L. cavernous sinuses. c and d, patient 5, axial and coronal plane imaging demonstrating spread along cranial nerve V in R. cavernous sinus. e and f, patient 1, axial and sagittal plane imaging demonstrating spread along R. cranial nerve V into orbit. Yellow line denotes the 50% isodose line. The green line represents the 30% isodose line. The green box denotes the target lesion of interest. The red lines denote the isodose plot.
Patient 3 followed a rapid course, likely due to the aggressiveness of SMARCB1-deficient sinonasal carcinoma, which is also discussed in a separate case report.[
Patient 5 did show some interval improvement in PNS after GKRS, however, osseous involvement of the tumor as well as extracranial tumor burden was likely the reason for uncontrolled progression. Extracranial progression evolved to include the infratemporal fossa, pterygoid muscles, parotid space, and parapharyngeal spaces. By this point, palliative chemotherapy was attempted but the patient was soon made comfort care.
Patient 1 presents a more complicated picture from benefits of GKRS (6/2014). MRI 2 months after GKRS did show resolution of some areas of previous enhancement and improvement in other area. MRI at 6 months post-GKRS then showed recurrent enhancing mass of PNS along the ophthalmic division of CN V. However, this patient had inconsistent attendance to appointment visits and missed multiple chemotherapy infusion between the time of recurrence and death.
Special considerations
Of the host of symptomatic manifestations that can accompany perineural disease, refractory trigeminal pain is one of the most morbid. Trigeminal neuralgia (TN), or Tic Douloureux, is infamous for its classic, characteristic excruciating pain – leading to the term “suicide disease” coined as early as the 18th century. In perineural disease, this brings up an interesting paradox from a diagnostic and treatment perspective. Even in early descriptions of PNS, paresthesia followed by pain was a characteristic sign of CN V involvement.[
Typically, a high dose of 70–80 Gy is delivered to the trigeminal root entry zone in treating nontumor TN, while lower doses of 18–22Gy are used for tumor-related TN. A recent series of patients, however, demonstrated that GKRS to both the tumor mass and the root zone provided more durable pain relief.[
Whether presenting with isolated TN, or TN secondary to perineural disease, etiology of pathology should be taken into account in decision-making for treatment. In secondary TN, the morbidity of the symptoms alone should be taken into account when determining treatment options. Further study is also warranted in this matter as not all patients with CN V involvement have TN symptoms. The patient with TN symptoms from PNS seems to be refractory to traditional TN treatment, however, combination targeting of both the tumor and trigeminal root zone looks to provide for durable pain relief.[
CONCLUSION
The role of PNI and spread in head-and-neck cancer remains an often confused, and underreported, phenomena. More insight into the unique pathophysiology behind this type of metastatic spread is warranted. Perineural disease remains difficult to treat and requires the expertise of a multidisciplinary team, including neurosurgeons; in this context, a proper assessment should not only evaluate the need, and morbidity, of surgical resection and radiosurgery but also the symptomatic manifestations such as trigeminal neuralgia. Goals of treatment should thus include minimizing treatment- related morbidity and toxicity while maintaining patient quality of life. In our series, GKRS proved to be a helpful utility in limiting or reducing tumor burden and also has a role for palliative use in reducing symptoms. Given the morbidity and mortality of head-and-neck cancer with PNS, time is limited, and multiple methods of treatment must be utilized.
To improve outcomes, education of PNS must be increased within specialties to aid in rapid recognition of perineural involvement and early involvement of a multidisciplinary treatment team. Further case series and reports on patients with PNS are essential to provide insight into disease course and pathophysiology. This and other previous series have been limited due to low incidence and PNS not being restricted to any specific cancer pathology. Further work in utilizing novel therapies such as checkpoint inhibitors will also likely aid in management of primary tumor burden and potentially prevent or limit PNI and spread.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amit M, Eran A, Billan S, Fridman E, Na’ara S, Charas T. Perineural spread in noncutaneous head and neck cancer: New insights into an old problem. J Neurol Surg B Skull Base. 2016. 77: 86-95
2. Balamucki CJ, Mancuso AA, Amdur RJ, Kirwan JM, Morris CG, Flowers FP. Skin carcinoma of the head and neck with perineural invasion. Am J Otolaryngol. 2012. 33: 447-54
3. Boerman RH, Maassen EM, Joosten J, Kaanders HA, Marres HA, van Overbeeke J. Trigeminal neuropathy secondary to perineural invasion of head and neck carcinomas. Neurology. 1999. 53: 213-6
4. Catalano PJ, Sen C, Biller HF. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol. 1995. 16: 772-7
5. Dhople AA, Adams JR, Maggio WW, Naqvi SA, Regine WF, Kwok Y. Long-term outcomes of Gamma Knife radiosurgery for classic trigeminal neuralgia: Implications of treatment and critical review of the literature. J Neurosurg. 2009. 111: 351-8
6. Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998. 124: 637-40
7. Fukai J, Fujita K, Yamoto T, Sasaki T, Uematsu Y, Nakao N. Intracranial extension of adenoid cystic carcinoma: Potential involvement of EphA2 expression and epithelial-mesenchymal transition in tumor metastasis: A case report. BMC Res Notes. 2014. 7: 131-
8. Gandhi MR, Panizza B, Kennedy D. Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: Comparing targeted MRI with the histologic findings following surgery. Head Neck. 2011. 33: 469-75
9. Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984. 148: 542-7
10. Gomez-Acevedo H, Patterson JD, Sardar S, Gokden M, Das BC, Ussery DW. SMARC-B1 deficient sinonasal carcinoma metastasis to the brain with next generation sequencing data: A case report of perineural invasion progressing to leptomeningeal invasion. BMC Cancer. 2019. 19: 1-10
11. Guss ZD, Batra S, Limb CJ, Li G, Sughrue ME, Redmond K. Radiosurgery of glomus jugulare tumors: A meta-analysis. Int J Radiat Oncol Biol Phys. 2011. 81: e497-502
12. Jackson JE, Dickie GJ, Wiltshire KL, Keller J, Tripcony L, Poulsen MG. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: Toward a risk-adapted treatment approach. Head Neck. 2009. 31: 604-10
13. Kim SK, Kim DG, Se YB, Kim JW, Kim YH, Chung HT. Gamma Knife surgery for tumor-related trigeminal neuralgia: Targeting both the tumor and the trigeminal root exit zone in a single session. J Neurosurg. 2016. 125: 838-44
14. Künzel J, Koch M, Brase C, Fietkau R, Iro H, Zenk J. Treatment of cervical paragangliomas: Is surgery the only way?. Am J Otolaryngol. 2014. 35: 186-91
15. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer. Cancer. 2009. 115: 3379-91
16. Lin C, Tripcony L, Keller J, Poulsen M, Dickie G. Cutaneous carcinoma of the head and neck with clinical features of perineural infiltration treated with radiotherapy. Clin Oncol. 2013. 25: 362-7
17. Panizza B, Solares CA, Redmond M, Parmar P, O’Rourke P. Surgical resection for clinical perineural invasion from cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2012. 34: 1622-7
18. Regis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J. Long-term safety and effiacy of Gamma Knife surgery in classical trigeminal neuralgia: A 497-patient historical cohort study. J Neurosurg. 2016. 124: 1079-87
19. Roh J, Muelleman T, Tawfik O, Thomas SM. Perineural growth in head and neck squamous cell carcinoma: A review. Oral Oncol. 2015. 51: 16-23
20. Skarsgard DP, Groome PA, Mackillop WJ, Zhou S, Rothwell D, Dixon PF. Cancers of the upper aerodigestive tract in Ontario, Canada, and the United States. Cancer. 2000. 88: 1728-38
21. Solares C, Mason E, Panizza B. Surgical management of perineural spread of head and neck cancers. J Neurol Surg B Skull Base. 2016. 77: 140-9
22. Sytnyk V, Leshchyns’ka I, Schachner M. Neural cell adhesion molecules of the immunoglobulin superfamily regulate synapse formation, maintenance, and function. Trends Neurosci. 2017. 40: 295-308
23. Tuleasca C, Carron R, Resseguier N, Donnet A, Roussel P, Gaudart J. Repeat Gamma Knife surgery for recurrent trigeminal neuralgia: Long-term outcomes and systematic review. J Neurosurg. 2014. 121: 210-21
24. Warren TA, Panizza B, Porceddu SV, Gandhi M, Patel P, Wood M. Outcomes after surgery and postoperative radiotherapy for perineural spread of head and neck cutaneous squamous cell carcinoma. Head Neck. 2016. 38: 824-31
25. Warren TA, Whiteman DC, Porceddu SV, Panizza BJ. Insight into the epidemiology of cutaneous squamous cell carcinoma with perineural spread. Head Neck. 2016. 38: 1416-20