- Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka, Japan.
- Department of Neurosurgery, NTT Medical Center Tokyo, Higashigotanda, Tokyo, Japan.
Correspondence Address:
Hideaki Ono
Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka, Japan.
DOI:10.25259/SNI_402_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yudai Hirano1, Hideaki Ono1, Tomohiro Inoue2, Toshiya Aono1, Takeo Tanishima1, Akira Tamura1, Isamu Saito1. Superficial temporal artery-superior cerebellar artery bypass and proximal occlusion through anterior petrosal approach for subarachnoid hemorrhage due to basilar artery dissection. 21-Aug-2020;11:256
How to cite this URL: Yudai Hirano1, Hideaki Ono1, Tomohiro Inoue2, Toshiya Aono1, Takeo Tanishima1, Akira Tamura1, Isamu Saito1. Superficial temporal artery-superior cerebellar artery bypass and proximal occlusion through anterior petrosal approach for subarachnoid hemorrhage due to basilar artery dissection. 21-Aug-2020;11:256. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10223
Abstract
Background: Subarachnoid hemorrhage (SAH) due to rupture of basilar artery dissection (BAD) is extremely rare and often has a poor prognosis. Since ruptured BAD has high rate of rebleeding and mortality, treatment to prevent rerupture is mandatory in the acute phase. However, to date, no optimal treatment has been established which satisfies secure prevention of rerupture and ischemia simultaneously. Herein, we report a case of SAH due to BAD treated with proximal occlusion of basilar artery with superficial temporal artery (STA)-superior cerebellar artery (SCA) bypass, preventing rebleeding securely and ensuring adequate blood flow in the upper basilar region.
Case Description: A 48-year-old male presenting with headache and altered mental status was found to have SAH and BAD. To prevent rerupture, proximal occlusion of basilar artery with STA-SCA bypass using anterior transpetrosal approach was performed. The postoperative course was relatively good and there is no evidence of recurrent arterial dissection.
Conclusion: Proximal occlusion of the basilar artery combined with STA-SCA bypass was successful in preventing rerupture of BAD and ensuring blood flow in the upper basilar region. Although there is controversy regarding acute treatment for ruptured BAD, direct proximal occlusion with sufficient revascularization using bypass skull base technique may be one of the optimal treatments even in this era of endovascular treatment.
Keywords: Basilar artery dissection, Bypass, Proximal occlusion, Superficial temporal artery-Superior cerebellar artery, Subarachnoid hemorrhage
INTRODUCTION
Basilar artery dissection (BAD) is very rare disease[
Here, we report a case of ruptured BAD treated by proximal occlusion of basilar artery in conjugation with superficial temporal artery (STA)-superior cerebellar artery (SCA) bypass using skull base technique in the acute phase.
CASE PRESENTATION
A 48-year-old man with no medical history was aware of intermittent headache 1 month before his visit. Suddenly, he woke up with a severe headache and did not show any improvement thereafter. During the emergency transportation, the state of consciousness deteriorated rapidly, and the Glasgow Coma Scale was detected as 7 (E1, V2, M4) on admission. Head computed tomography (CT) on admission revealed diffuse subarachnoid hemorrhage, especially thick in prepontine cistern (Fisher group 3), complicated by hydrocephalus [
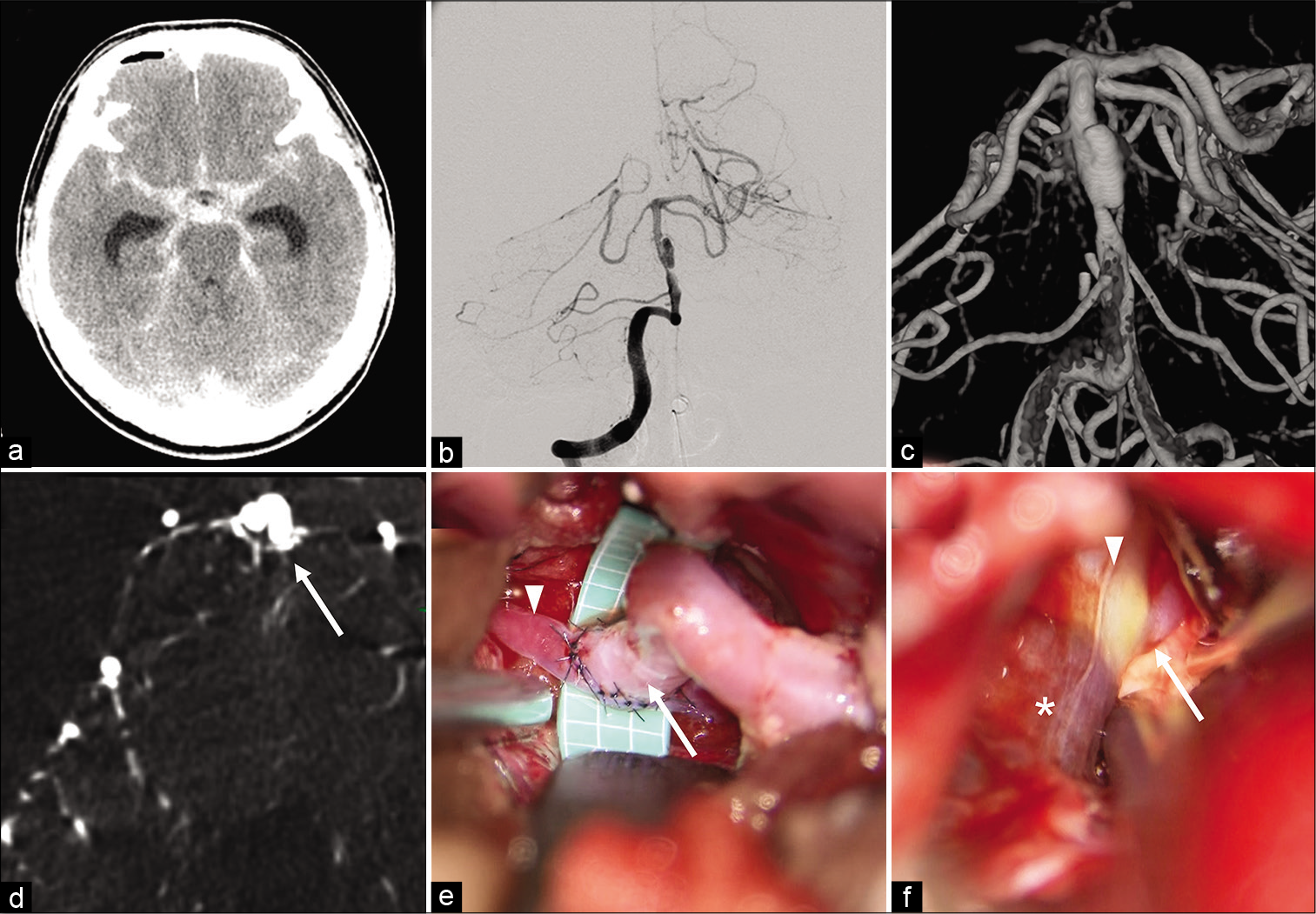
Figure 1:
Preoperative imaging and intraoperative view. (a) Preoperative computed tomography scan showing a massive subarachnoid hemorrhage. (b) anteroposterior view of vertebral angiography showing spindle shaped aneurysmal changes of basilar artery (BA) between the bifurcations with the superior cerebellar artery (SCA) and anterior inferior cerebellar artery (AICA). (c) intimal flap and double-lumen sign were confirmed by 3-dimentional digital subtraction angiography (3D-DSA). (d) high-resolution CT showing perforators (arrow) originating from the dissecting artery. (e) intraoperative view: superficial temporal artery (arrow) -superior cerebellar artery (arrowhead) bypass. (f) intraoperative view: BA just proximall to origin of AICA (arrow) was almost normal (arrowhead) and there were no perforators nearby. BA distal to AICA was dissected (star).
In this case, since BAD was in the acute stage of rupture, blocking the blood flow to the dissecting part was necessary to prevent rerupture. It was difficult to clip directly considering the shape of a dissecting aneurysm. Furthermore, trapping the lesion might cause brainstem infarction since the high- resolution CT showed perforators originating from the dissecting artery [
Thus, we planned proximal ligation of BAD by direct clip just distal to AICA and STA-SCA bypass, intended to prevent repeated hemorrhage from BAD by blocking anterograde blood flow, preserve blood flow to AICA, and maintain adequate blood flow in upper BA including perforators originated around BAD.
Operation
Neuroanesthesia was induced and the patient was set in the supine-lateral position. Somatosensory evoked potentials of the four limbs, right auditory brainstem response, and right facial nerve function were monitored. The STA was harvested under the operating microscope. After cutting the zygoma and moving it downward with temporal muscle, a temporal craniotomy was performed with a 4-burr hole. The temporal base was flattened, and the middle fossa dura was detached from the middle skull base. The foramen spinosum was identified and the middle meningeal artery was coagulated and transected. Anterior petrosectomy was conducted by drilling the Kawase’s triangle which is surrounded by the greater superficial petrosal nerve, arcuate eminence, and the lateral edges of mandibular nerve[
Postoperative course
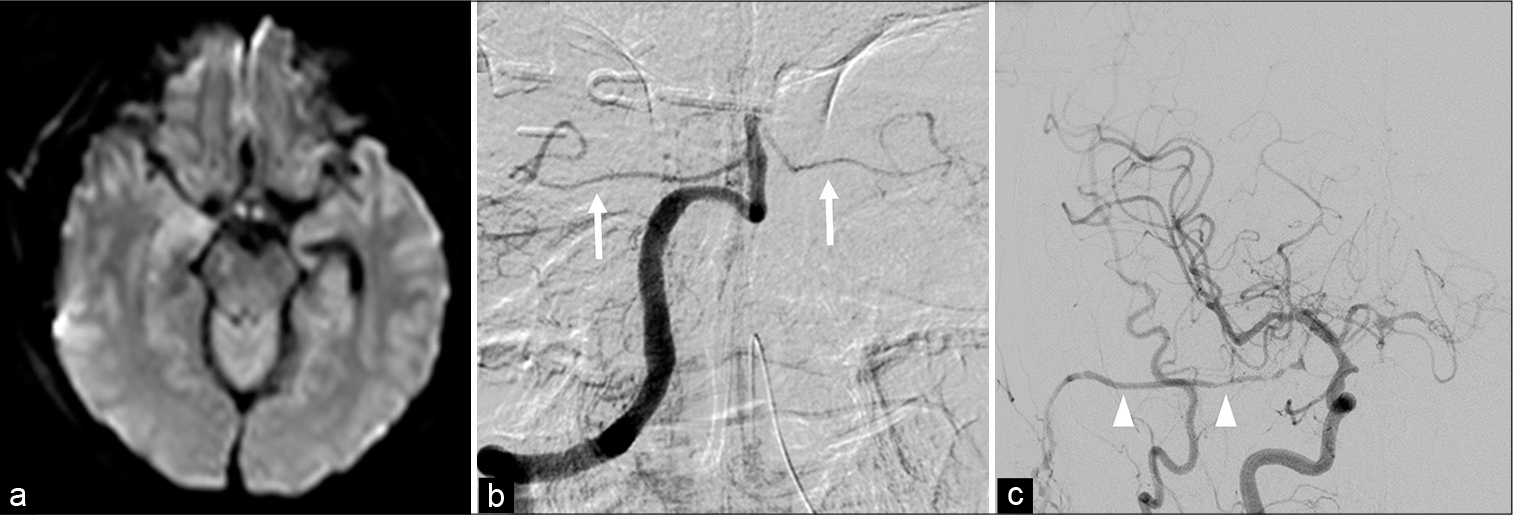
The postoperative course was relatively fair. To prevent obstruction of the bypass blood vessel, oral administration of aspirin (100 mg/day) was started. Postoperative diffusion-weighted magnetic resonance (MR) imaging showed an infarction in right midbrain which causes partial paralysis of the left limb [
DISCUSSION
BAD is a very rare disease, as intracranial arterial dissection tends to occur mostly in vertebral artery according to large case series[
In cases of BAD without hemorrhage or infarction, conservative treatment with strict control of blood pressure is considered. On the other hand, the morbidity and mortality rate of ruptured BAD is 20% and 40%, respectively, which are much higher than those of unruptured BAD[
Direct surgery for dissecting aneurysms of the posterior circulatory system is generally difficult and may have serious consequences. There are few reports that direct surgery worked well in BAD[
In recent years, with the advancement of new devices, the number of cases of endovascular treatment for BAD has been increasing. In addition to coils and conventional stents[
However, there are several problems in endovascular treatment. First, anticoagulation and dual antiplatelet therapy induction are necessary in acute phase of rupture to prevent thromboembolic complications including occlusion. Second, flow alteration with stent or flow diverters, unlike clipping, does not completely prevent blood flow to BAD, so there is a risk of rebleeding even after treatment during the acute phase of rupture. It takes about 1 month to complete the regeneration of the vascular endothelium or intimal thickening in the part of dissection[
The purpose of proximal occlusion for BAD is to block anterograde blood flow to the dissection. Clipping is possible for saccular type aneurysms, and only two cases have been reported in the past[
The important point in proximal occlusion of the parent artery is to preserve sufficient blood flow to distal part of the artery. Although blood flow to PCA is supplied by cross flow from the anterior circulation through right PCoA in this case, we could not confirm that the flow is sufficient. To examine whether the blood flow through PCoA would replace upper BA or not, meticulous collateral evaluation by balloon occlusion test should have been performed. However, we did not perform the procedure due to patient’s deteriorated consciousness and risk of thromboembolic complications. Thus, we tried to maintain blood flow by conducting STA- SCA bypass together [
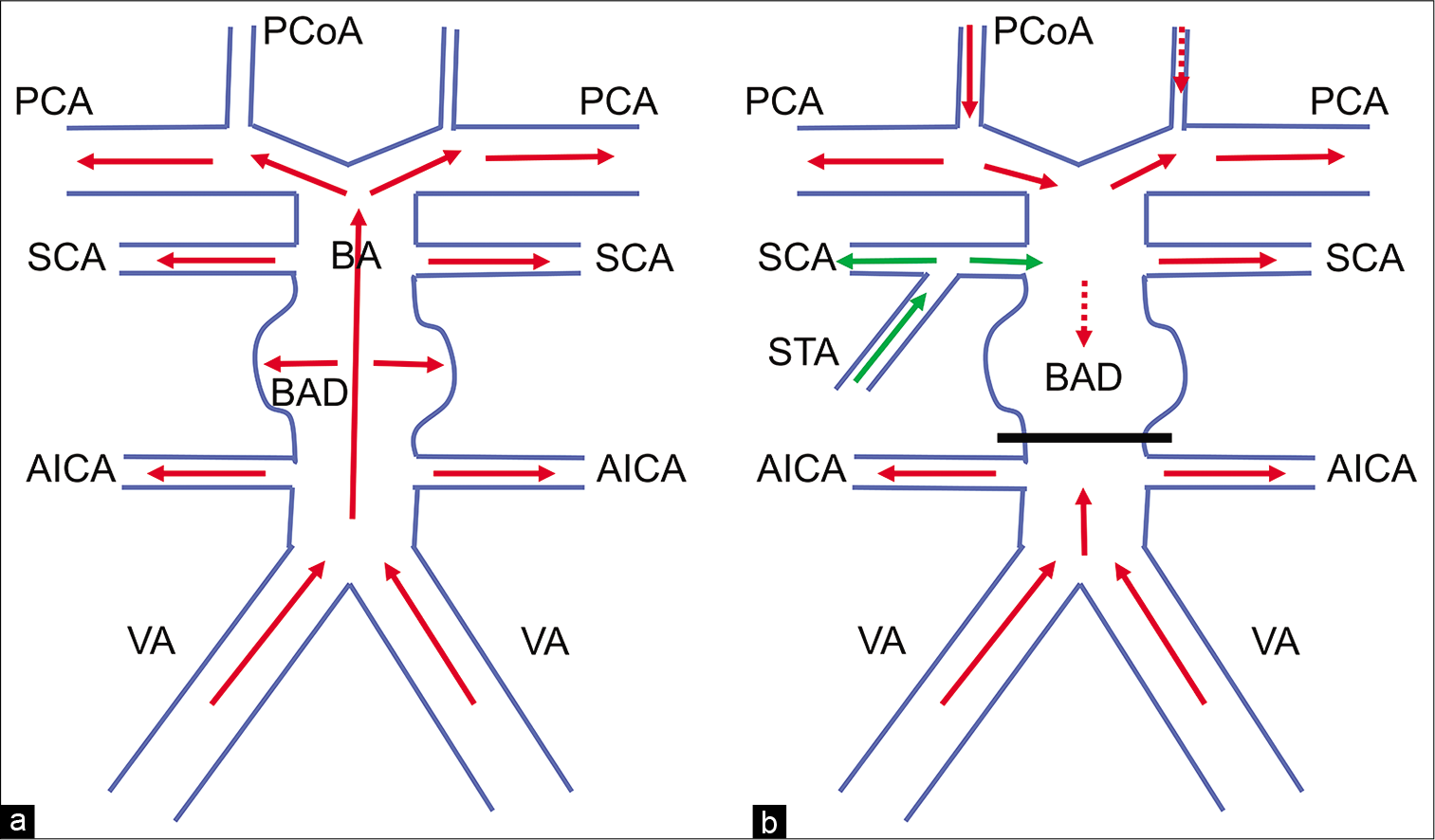
Figure 3:
Schematic drawings of the surgery for the basilar artery dissection. (a,b) Preoperative and postoperative hemodynamics. Postoperatively, using superior temporal artery-superior cerebellar artery (SCA) bypass together with clipping just the distal part of anterior inferior cerebellar artery, the blood flow of SCA is covered by the bypass. The blood flow of posterior cerebral artery is supplied from the anterior circulation through posterior communicating artery. There is no blood flow in the dissection. Red arrows: normal blood flow, green arrows: blood flow by STA-SCA bypass, VA: vertebral artery.
The postoperative course was fairly good, with no apparent complications other than the right midbrain infarction.
Because the infarction occurred in the right midbrain, not in the pons, we assumed the causes as that the hemodynamic change after the operation caused insufficiency of blood flow to the perforators to midbrain and that the cisternal drain compressed the midbrain itself or small artery around[
There is no standard treatment for ruptured BAD, and optimal treatment should be provided according to each patient and condition to prevent rebleeding. In addition to blocking the blood flow to the dissecting part, it is effective to use a bypass technique together to secure the blood flow to the area where ischemia may occur.
CONCLUSION
There is no standard treatment for ruptured BAD, and optimal treatment should be provided according to each patient. Proximal occlusion of the BAD combined with STA- SCA bypass was successful in preventing rerupture of BAD and ensuring blood flow in the upper BA region. Although there is controversy regarding acute treatment for ruptured BAD, direct surgery using bypass and skull base technique may be one of the optimal treatments.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Ali MJ, Bendok BR, Tella MN, Chandler JP, Getch CC, Batjer HH. Arterial reconstruction by direct surgical clipping of a basilar artery dissecting aneurysm after failed vertebral artery occlusion: Technical case report and literature review. Neurosurgery. 2003. 52: 1475-80
2. Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990. 21: 1628-31
3. Defillo A, Nussbaum ES, Zelensky A, Nussbaum L. Multiple non-branching dissecting aneurysms of the mid-basilar trunk presenting with sequential subarachnoid hemorrhages. Surg Neurol Int. 2011. 2: 127
4. Endo H, Matsumoto Y, Kondo R, Sato K, Fujimura M, Inoue T. Medullary infarction as a poor prognostic factor after internal coil trapping of a ruptured vertebral artery dissection. J Neurosurg. 2013. 118: 131-9
5. Horiuchi T, Yamamoto Y, Kuroiwa M, Rahmah NN, Hongo K. Pontine infarction caused by medial branch injury of the basilar artery as a rare complication of cisternal drain placement. J Clin Neurosci. 2012. 19: 592-3
6. Jiang C, Li Q, Liu JM, Huang QH. Endovascular treatment for the basilar artery dissection. Cardiovasc Intervent Radiol. 2014. 37: 646-56
7. Kawase T, Toya S, Shiobara R, Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985. 63: 857-61
8. Kim BM, Suh SH, Park SI, Shin YS, Chung EC, Lee MH. Management and clinical outcome of acute basilar artery dissection. AJNR Am J Neuroradiol. 2008. 29: 1937-41
9. Koizumi S, Shojima M, Iijima A, Oya S, Matsui T, Yoshikawa G. Stent-assisted coiling for ruptured basilar artery dissecting aneurysms: An initial experience of four cases. Neurol Med Chir (Tokyo). 2016. 56: 43-8
10. Kulcsar Z, Ernemann U, Wetzel SG, Bock A, Goericke S, Panagiotopoulos V. High-profile flow diverter (silk) implantation in the basilar artery: Efficacy in the treatment of aneurysms and the role of the perforators. Stroke. 2010. 41: 1690-6
11. Lehto H, Niemela M, Kivisaari R, Laakso A, Jahromi BR, Hijazy F. Intracranial vertebral artery aneurysms: Clinical features and outcome of 190 patients. World Neurosurg. 2015. 84: 380-9
12. Lim YC, Kim BM, Suh SH, Jeon P, Kim SH, Ihn YK. Reconstructive treatment of ruptured blood blister-like aneurysms with stent and coil. Neurosurgery. 2013. 73: 480-8
13. Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011. 114: 1037-44
14. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995. 36: 905-11
15. Mizutani T, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2004. 54: 342-7
16. Nakahara T, Satoh H, Mizoue T, Kawamoto H, Kohmo Y, Kurisu K. Dissecting aneurysm of basilar artery presenting with recurrent subarachnoid hemorrhage. Neurosurg Rev. 1999. 22: 155-8
17. Nakajima H, Ishiguro T, Komiyama M. Basilar artery dissection presenting with subarachnoid hemorrhage: Report of two cases. NMC Case Rep J. 2015. 2: 97-100
18. Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y. Symptomatic recurrence of intracranial arterial dissections: Follow-up study of 143 consecutive cases and pathological investigation. Stroke. 2013. 44: 126-31
19. Ramgren B, Cronqvist M, Romner B, Brandt L, Holtas S, Larsson EM. Vertebrobasilar dissection with subarachnoid hemorrhage: A retrospective study of 29 patients. Neuroradiology. 2005. 47: 97-104
20. Ruecker M, Furtner M, Knoflach M, Werner P, Gotwald T, Chemelli A. Basilar artery dissection: Series of 12 consecutive cases and review of the literature. Cerebrovasc Dis. 2010. 30: 267-76
21. Saliou G, Power S, Krings T. Flow diverter placement for management of dissecting ruptured aneurysm in a non-fused basilar artery. Interv Neuroradiol. 2016. 22: 58-61
22. Sonobe S, Yoshida M, Niizuma K, Tominaga T. Ruptured basilar artery dissection diagnosed using magnetic resonance vessel wall imaging and treated with coil embolization with overlapping LVIS stents: A case report. NMC Case Rep J. 2020. 7: 75-9
23. Van Oel LI, Van Rooij WJ, Sluzewski M, Beute GN, Lohle PN, Peluso JP. Reconstructive endovascular treatment of fusiform and dissecting basilar trunk aneurysms with flow diverters, stents, and coils. AJNR Am J Neuroradiol. 2013. 34: 589-95
24. Yamaura A, Ono J, Hirai S. Clinical picture of intracranial non-traumatic dissecting aneurysm. Neuropathology. 2000. 20: 85-90
25. Yoshimoto Y, Hoya K, Tanaka Y, Uchida T. Basilar artery dissection. J Neurosurg. 2005. 102: 476-81






Jim Ausman
Posted September 8, 2020, 12:09 pm
Excellent surgery and plan. You might be interested in my paper on the first use of STA to SCA bypass Ausman JI, Lee MC, Chater N, Latchaw RE: Superficial temporal artery to superior cerebellar artery anastomosis for distal basilar artery stenosis. Surg Neurol, 12:277282, 1979.