- Department of Hematology Bab Saadoun, Tunis, Tunisia.
- Department of Neurosurgery, Military Hospital of Tunis, Bab Saadoun, Tunis, Tunisia.
- Department of Radiotherapy, Salah Azaiez Institue, Bab Saadoun, Tunis, Tunisia.
- Department of Hematology, Aziza Othmana University Hospital, Faculty of Medecine of Tunis, University Tunis El Manar, Bab Saadoun, Tunis, Tunisia.
Correspondence Address:
Khaled Radhouane
Department of Neurosurgery, Military Hospital of Tunis, Bab Saadoun, Tunis, Tunisia.
DOI:10.25259/SNI_592_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hela Ghedira1, Khaled Radhouane2, Essia Mezni1, Safia Yahiaoui3, Hela Stambouli1, Mohamed Dehmani Yedeas2, Asma Belaid3, Chiraz Ammar3, Karima Kacem4, Samy Zriba1, Fehmi Msadek1, Mondher Yedeas2, Ahmed Harbaoui2, Ridha Chkili2. Impact of local treatment on survival from hematological malignancies causing spinal cord compression. 21-Oct-2020;11:352
How to cite this URL: Hela Ghedira1, Khaled Radhouane2, Essia Mezni1, Safia Yahiaoui3, Hela Stambouli1, Mohamed Dehmani Yedeas2, Asma Belaid3, Chiraz Ammar3, Karima Kacem4, Samy Zriba1, Fehmi Msadek1, Mondher Yedeas2, Ahmed Harbaoui2, Ridha Chkili2. Impact of local treatment on survival from hematological malignancies causing spinal cord compression. 21-Oct-2020;11:352. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10343
Abstract
Background: Various hematological malignancies, including multiple myeloma, plasmacytoma, aggressive lymphoma, and indolent lymphoma, rarely result in spinal cord compression.
Methods: Here, we retrospectively analyzed 32 patients with multiple myeloma (50%), plasmacytoma (13%), aggressive lymphoma (28%), and indolent lymphoma (9%), resulting in spinal cord compression (2004 and 2016). Patients averaged 57 years of age and presented with the indolent onset of spinal cord compression (91% of cases) resulting mostly in motor deficits (69%).
Results: Local treatment modalities included radiotherapy (RT) (28%) alone, decompressive surgery (28%) alone, or decompressive surgery with consolidation RT (40%). The 1-year overall survival was 70%, and the progression-free survival frequency was 62%.
Conclusion: This study highlighted the importance of standardizing the indications for RT alone versus RT with surgery depending on the patient’s underlying pathological diagnosis, neurological deficits, and radiological findings.
Keywords: Chemotherapy, Hematological malignancy, Radiotherapy, Spinal cord compression, Surgery
INTRODUCTION
Malignant cord compression (MCC) may occur due to hematological malignancies (HMs) that may be responsive to chemotherapy and/or radiation therapy. The incidence of MCC in HMs ranges between 6 and 20% and is just behind the frequency of solid neoplasms. Except for multiple myeloma (MM: 5%) and lymphoma (6.5%), the actual incidence of MCC as the initial presentation of the HM is unknown. Due to the rarity of HMs with MCC, it is difficult to determine the optimal schedule of treatment.
Here, we evaluated the epidemiological, clinical, and therapeutic characteristics of patients with HM resulting in MCC in an effort to identify therapeutic and prognostic risks factors for attaining the best outcomes.
MATERIALS AND METHODS
We retrospectively studied 431 patients treated for HM, in the department of clinical hematology (2004–2016). The diagnosis of MCC was confirmed radiologically utilizing MR imaging and/or CT scans. In addition, the diagnoses of HM were histologically confirmed for all cases of lymphoma, and cytologically, and/or histologically for MM/ plasmacytoma.
Treatment and follow-up
We reviewed the results of treatment utilizing radiotherapy (RT) alone, RT with surgery, with/without chemotherapy (CT), for the management of MCC due to HM. Conventional 2D RT (normofractionated or hypofractionated RT) was used in all irradiated patients and was either immediately after the diagnosis of MCC/HM was established, or just after surgical decompression (minimal laminectomy) was performed. All patients immediately received dexamethasone; the dosage was 10 mg twice a day, followed by 4 mg 4 times a day during 3–7 days, with a gradual tapering off within 2 weeks.
Systemic treatment
Systemic treatment modalities included chemotherapy (CT), immunomodulatory therapies, and zoledronic acid treatment. Some were utilized for the additional treatment of select patients with MCC (except in case of solitary plasmacytoma). Follow-up visits were performed at 1, 3, 6, and 12 months after discharge and then annually.
Data
Multiple clinicopathological data and treatment decisions (RT alone, surgery alone, and surgery/RT with/without CT) were assessed by reviewing electronic medical records. Outcomes were analyzed for overall survival (OS), progression-free survival (PFS), and to determine the efficacy of local RT versus no RT, surgery versus no surgery, and surgery followed by RT versus RT alone, and surgery alone.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 22.0; OS was defined as the time from the first therapeutic action to death, while PFS was the time from the first therapeutic action progression confirmed radiologically or cytologically and/or histologically or death. Survival analyses included Cox proportional hazard function, and the Kaplan–Meier plot survival curves, and log-rank test to assess prognostic factors.
Materials: clinical data
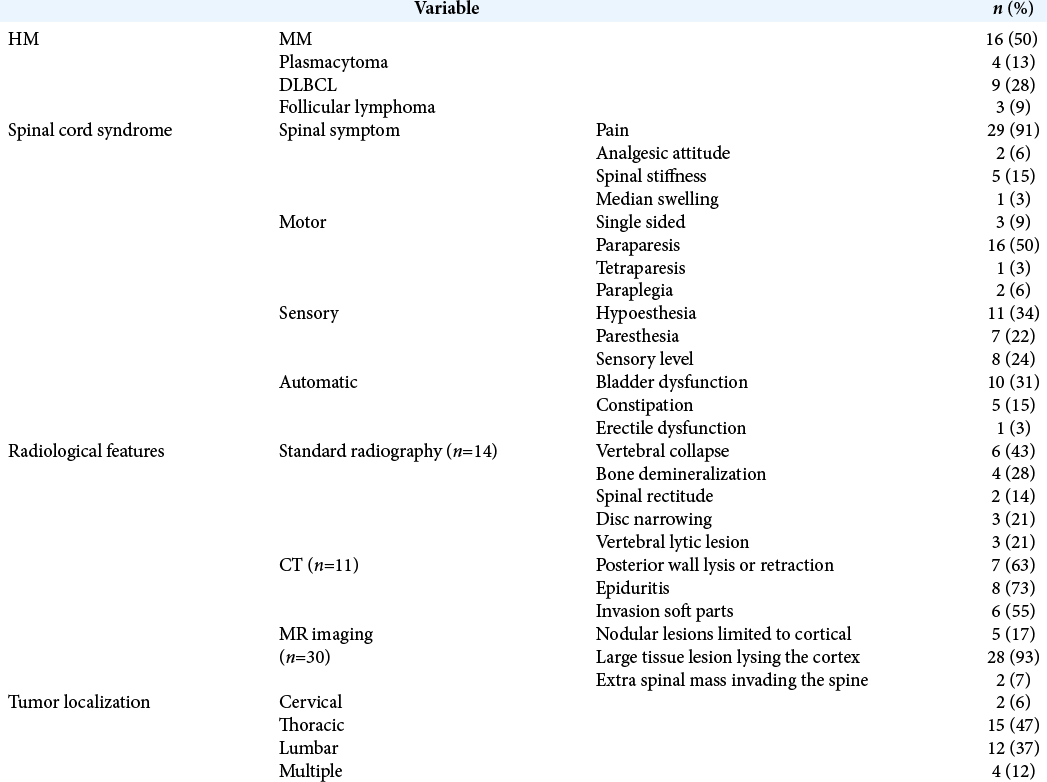
Our cohort included 32 patients averaging 57 years of age. They had the following pathological diagnosis attributed to HM: multiple myeloma MM (n = 16), plasmacytoma (n = 4), diffuse large B-cell lymphoma (DLBCL) (n = 9), and follicular lymphoma (n = 3). The median time from the onset of symptoms to treatment for spinal cord compression was 10 weeks. Accompanying clinical features included paralysis (50%), sensory levels (24%), and bladder dysfunction (31%) [
RESULTS
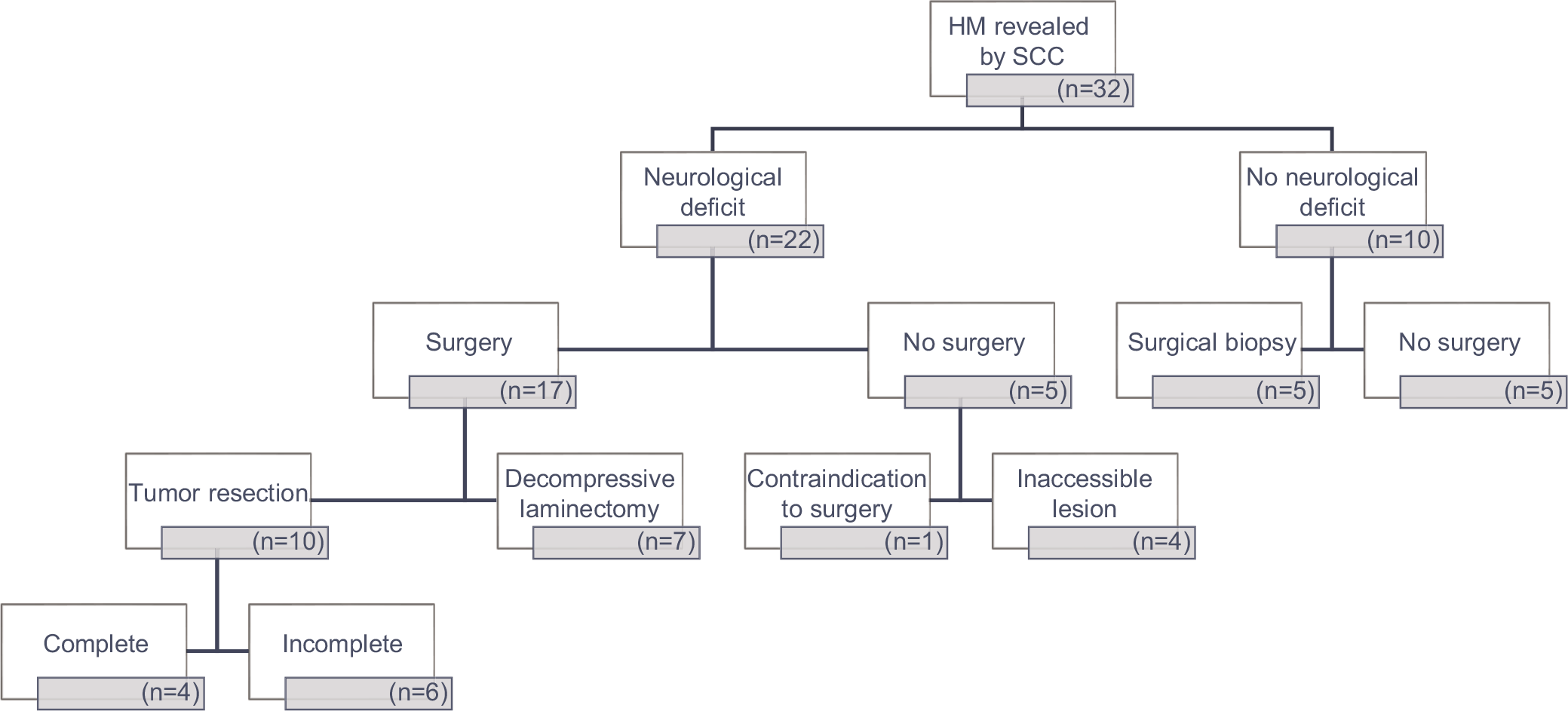
Surgery
Twenty-two patients (69%), 17 with preoperative neurological deficits, underwent posterior surgery [
Radiotherapy
Twenty-two patients (69%) had spinal radiotherapy; 9 had RT alone, while 13 had RT with surgery. For the 9 patients who had RT alone, 6 received a normofractionated regimen (2 Gray (Gy) × 22 or 3 Gy × 10), while 3 had hypofractionated treatment (3 or 4 Gy ×5). Ten of the 13 who underwent consolidation RT following a surgical decompression received normofractionated radiotherapy (3 Gy × 10).
Systemic treatment: chemotherapy and immunotherapy
Twenty-nine patients (90%) received systemic therapy including either chemotherapy or immunomodulatory treatments that differed according to the various types of tumors encountered. Two patients with solitary bone plasmacytoma did not require systemic treatment and one patient with follicular lymphoma was lost to follow-up after decompressive surgery and consolidation radiotherapy. The median time from spinal cord compression to specific systemic treatment was 7 weeks (3–16 weeks).
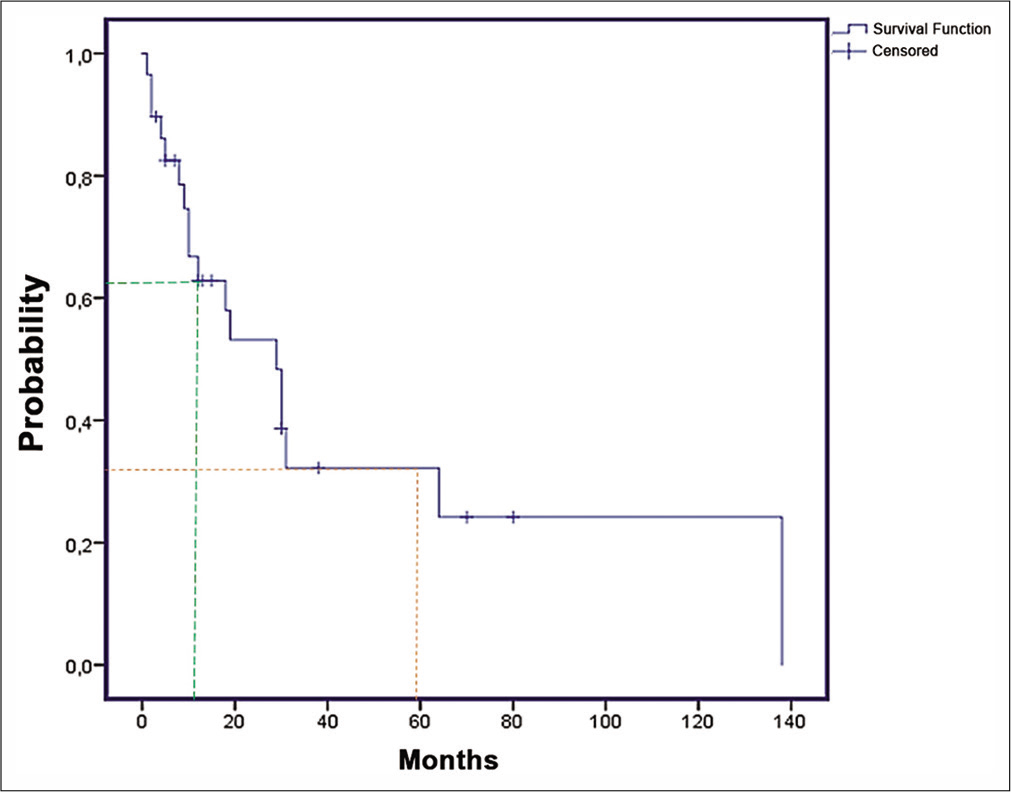
Survival and prognostic factors
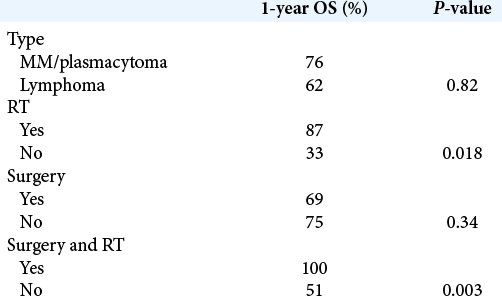
There was a significantly higher survival probability when RT was used and when surgery was associated with consolidation RT [
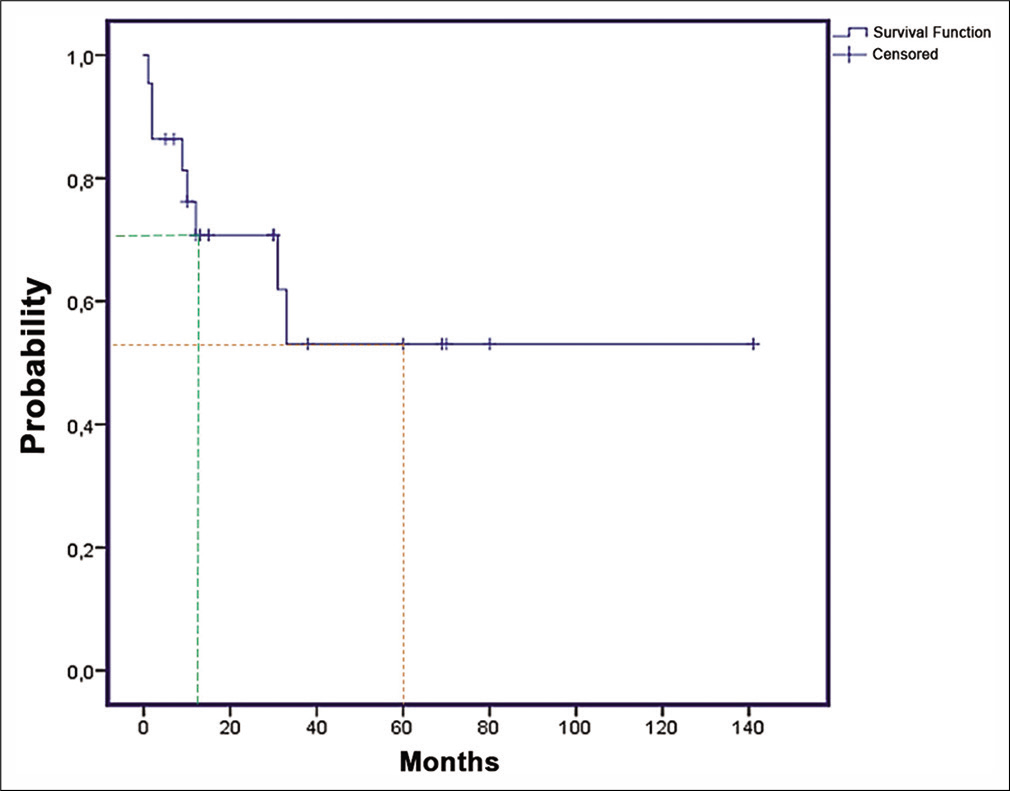
The 1-year and 5-year OS were, respectively, 70% and 53% [
DISCUSSION
Due to their heterogeneity and rarity, controlled clinical trials in MCC/HMs are rare. Recommendations are largely based on (retrospective) cohort studies and expert opinions. However, the therapeutic approach should alternatively come from multidisciplinary teams and should include RT alone, RT with surgery, and both with/without CT.
Frequency and indications for surgery
The use of surgery was more frequently utilized in our series as compared with the spinal literature. According to Flouzat et al. and Kim et al., patients with spinal cord compression secondary to HM were operated on in 25% and 8% of cases, respectively.[
In this study, our high 69% (22/32) frequency of surgery was attributed both to the increased frequency of significant initial/presenting neurological deficits and the need for pathological diagnoses. However, such tumor volume reduction surgery has limited utility in the management of asymptomatic patients with known hematological malignancies (especially for those who are extremely sensitive to RT and/or chemotherapy: MM [high-dose steroids and RT], plasmacytoma, and select lymphomas).[
Radiation therapy
Several regimens of RT can be used for patients with MCC: the hypofractionated schema (8 Gy in one fraction or 20 Gy in 5 fractions) and the standard schema with extended cycles (30 Gy in 10 fractions, or 37 Gy in 15 fractions, or 40 Gy in 20 fractions). In North America, a total dose of 30 Gy in 10 fractions is most frequently utilized while in Europe, a shorter schema (8 Gy or 20 Gy in 5 fractions) is preferred.[
CONCLUSION
The contribution of radiotherapy remains essential in improving the prognosis in MCC due to HMs. However, the indication for surgery depends on the presenting neurological deficit severity, accessibility of the lesion, and whether or not there is present or anticipated future spinal instability.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Flouzat-Lachaniette CH, Allain J, Roudot-Thoraval F, Poignard A. Treatment of spinal epidural compression due to hematological malignancies: A single institution’s retrospective experience. Eur Spine J. 2013. 22: 548-55
2. Kim SI, Kim YH, Ha KY, Lee JW, Lee JW. Surgical Roles for spinal involvement of hematological malignancies. J Korean Neurosurg Soc. 2017. 60: 534-9
3. Kwok Y, Tibbs PA, Patchell RA. Clinical approach to metastatic epidural spinal cord compression. Hematol Oncol Clin North Am. 2006. 20: 1297-305
4. Tsagozis P, Bauer HCF. Outcome of surgical treatment for spinal cord compression in patients with hematological malignancy. Int J Spine Surg. 2019. 13: 186-91