- Department of Ortho-Spine Surgery, Sir Ganga Ram Hospital, New Delhi, India.
- Department of Pathology, Sir Ganga Ram Hospital, New Delhi, India.
Correspondence Address:
Nitin Adsul
Department of Ortho-Spine Surgery, Sir Ganga Ram Hospital, New Delhi, India.
DOI:10.25259/SNI_515_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ratish Mishra1, Vishnu Prasad Panigrahi1, Nitin Adsul1, Sunila Jain2, R. S. Chahal1, K. L. Kalra1, Shankar Acharya1. Tophaceous gout in thoracic spine mimicking meningioma: A case report and literature review. 29-Oct-2020;11:364
How to cite this URL: Ratish Mishra1, Vishnu Prasad Panigrahi1, Nitin Adsul1, Sunila Jain2, R. S. Chahal1, K. L. Kalra1, Shankar Acharya1. Tophaceous gout in thoracic spine mimicking meningioma: A case report and literature review. 29-Oct-2020;11:364. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10359
Abstract
Background: Gout is a common metabolic disorder of purine metabolism, causing arthritis in the distal joints of the appendicular skeleton. Spine involvement is rare, and very few cases of spinal gout have been reported. The authors present a rare case of axial gout with tophaceous deposits in the thoracic spinal canal resulting in cord compression and mimicking a meningioma.
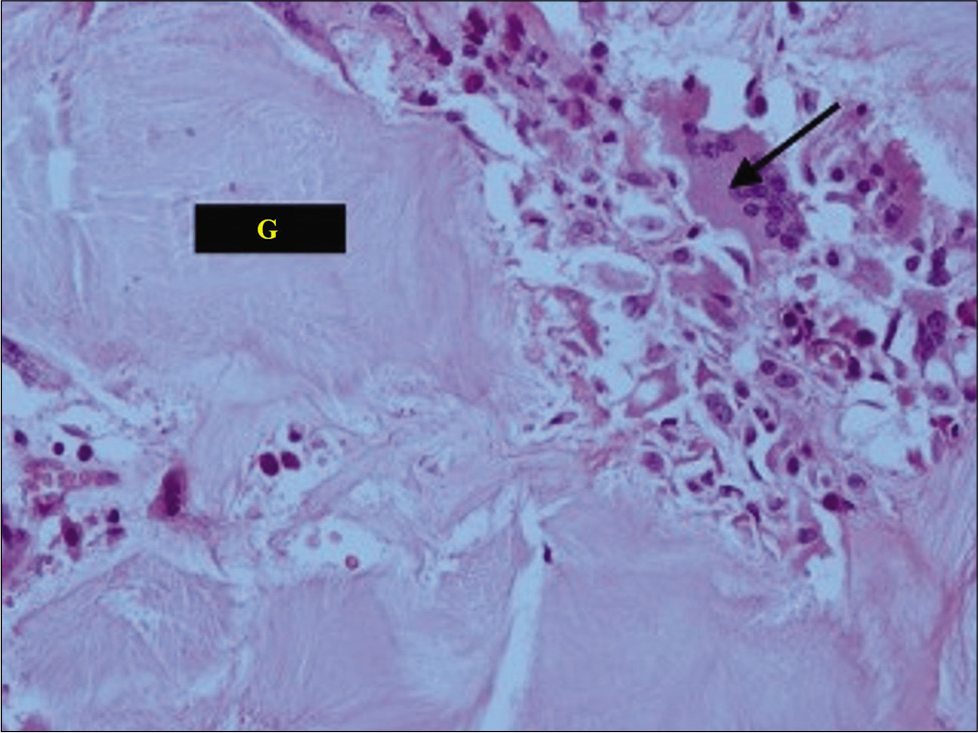
Case Description: A 33-year-old male presented with chronic mid back pain and a progressive paraparesis. The presumed diagnosis was meningioma based on MR imaging with/without contrast that showed a posterolateral, right-sided, and T10-T11 intradural extramedullary lesion. Notable, was hyperuricemia found on hematological studies. The patient underwent a decompressive laminectomy (T9-T11) for excision of the lesion, intraoperatively, an intraspinal, chalky, white mass firmly adherent to and compressing the dural sac was removed. The histopathology confirmed the diagnosis of a gouty tophus. Postoperatively, the patient’s pain resolved, and he regained the ability to walk.
Conclusion: A gouty tophus should be included among the differential diagnostic considerations when patients with known hyperuricemia present with back pain, and paraparesis attributed to an MR documented compressive spinal lesion.
Keywords: Axial gout, Meningioma, Spinal gout, Thoracic spinal gout, Tophaceous gout
INTRODUCTION
Gout is a common and complex form of arthritis characterized by classic signs of inflammation (i.e., dolor [pain], rubor [redness], calor [heat], and tumor [swelling] in the joint). Spinal involvement is rare, and very few cases of spinal gout have been reported in the literature.[
Here, we present here a case in which tophaceous deposits in the thoracic spinal canal mimicked a meningioma. We will also review the 25 similar cases of thoracic spinal gout reported in the literature.
CASE REPORT
Clinical presentation
A 33-year-old male presented with 9 months of back pain and 5 months of a progressive paraparesis that markedly worsened within the 5 days before admission. On examination, he had a paraparesis (right side [
Laboratory investigation and imaging
The patient’s total leucocyte cell count was increased to 22,290/mm3, the serum creatinine was high at 1.82 mg/dL, and the serum uric acid level was elevated to 11.4mg/dL. Notably, urine routine microscopy was normal and showed no “gouty” crystals.
Diagnostic studies
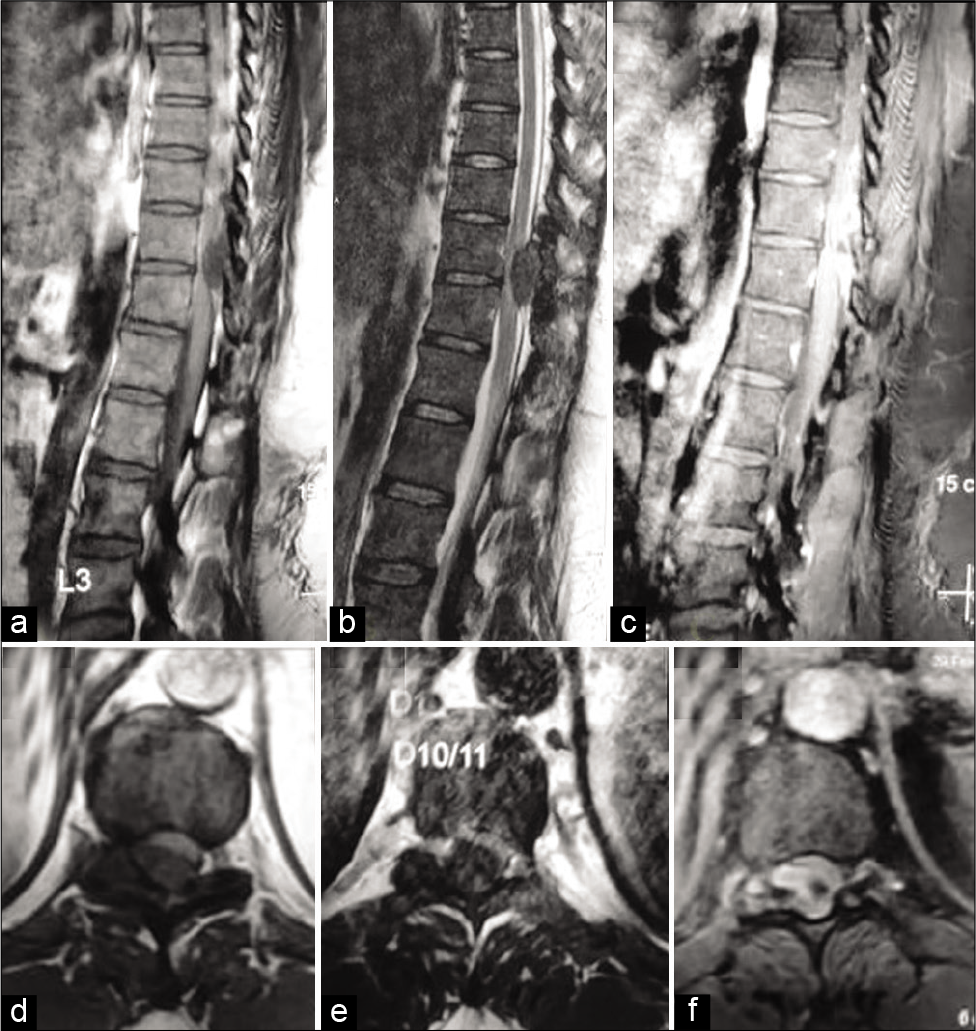
Although the thoracic x-rays were normal, the MR showed a posterolateral right-sided lesion at the T10-T11 level. The vertebral/intracanalicular lesion was iso- to hypointense on T1W images and heterogeneous/low signal intensity on T2W images; it was also accompanied by a focal hyperintense cord signal [
Figure 1:
Magnetic resonance imaging of thoracic spines. (a) Sagittal plane T1-weighted section. (b) T2-weighted section. (c) Contrast-enhanced T1-weighted section. (d) Axial plane T1-weighted section. (e) T2-weighted section. (f) Contrast-enhanced T1-weighted section. The images show an oval extramedullary intradural mass lesion (3.0 × 1.6 cm in size) at T10-11 lying to the right posterolateral aspect of the spinal cord. The lesion shows heterogeneous low signal intensity on T2W images and iso- to low signal intensity on T1W images with moderate heterogeneous enhancement.
Surgery
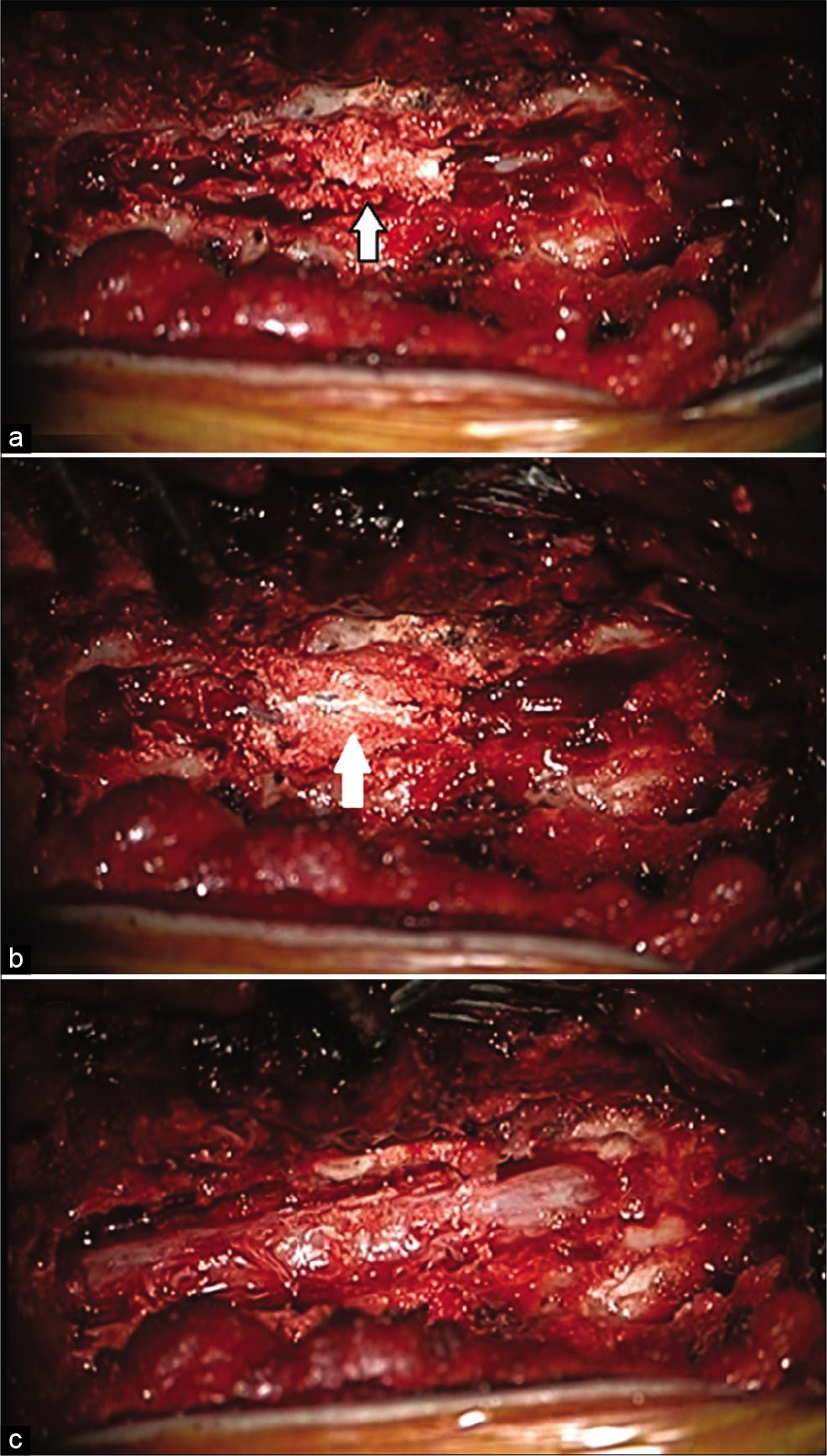
The patient underwent a T9 to T11 laminectomy. At surgery, the lesion was chalky/white, invaded the ligamentum flavum, adhered to the dura mater, and compressed the cord. [
Video 1
Outcome
Postoperatively, the patient’s pain was resolved, and his neurological deficit improved. He was able to walk within 3 postoperative months as his motor examination in both lower extremities improved bilaterally to the 4/5 level. He was subsequently referred to a rheumatologist for further management of his gout.
DISCUSSION
We identified 25 similar cases of spinal tophaceous gout reported in the literature. As these lesions are rare and can mimic spondylitis, neoplasm, or abscess; a histopathological examination is critical for establishing the correct diagnosis and determining the appropriate treatment.
Prior cases of thoracic spine gout
Axial gout is a disease of middle-aged men (76%), with most cases occurring between the ages of 44 and 74; females are less affected as estrogen lowers uric acid levels.[
Toprover et al.[
In most cases, patients have a history of prior gouty attacks, hyperuricemia, and/or renal failure. Toprover et al.[
In our case, hyperuricemia was detected on preoperative investigations without any known prior history of gout or hyperuricemia.
Diagnostic imaging
Imaging, including either X-rays or MR (with/without contrast), is typically nondiagnostic for differentiating spinal tophaceous gout from other lesions. On MR, a tophus may appear hypointense/isointense on T1, which may show variable intensity on T2, while contrast studies may demonstrate homogeneous/heterogeneous peripheral enhancement.[
Dual-energy CT (DECT)
DECT is a promising, noninvasive modality for the identification and volumetric quantification of tophaceous gout. It is both sensitive and specific for diagnosing gout and readily distinguishes urate crystals from calcium using specific attenuation characteristics. In patients with known tophaceous gout, it can be used for serial volumetric quantification of tophi to assess response to treatment.[
Management of gout
Management of gout includes treatment of the acute attack, lowering uric acid levels to prevent additional flare-ups of gouty arthritis, and/or the further deposition of urate crystals. Acute medical treatment includes the administration of colchicine, nonsteroidal anti-inflammatory drugs, or both, while long-term therapy mandates urate-lowering therapy (e.g., allopurinol, febuxostat, or probenecid).[
CONCLUSION
When patients with gouty arthritis or known hyperuricemia experience the new onset of neurological symptoms/signs in the presence of a spinal lesion, spinal tophaceous gout should be considered among the differential diagnostic considerations, warranating appropriate surgical management with the pathological confirmation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
Acknowledgment
We would like to acknowledge our senior pathologist, Prof. Sunila Jain, for the histopathological diagnosis of this patient.
References
1. Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh). 1977. 85: 198-208
2. Davies J, Riede P, Van Langevelde K, Teh J. Recent developments in advanced imaging in gout. Ther Adv Musculoskelet Dis. 2019. 11: 1759720X19844429
3. Hou LC, Hsu AR, Veeravagu A, Boakye M. Spinal gout in a renal transplant patient: A case report and literature review. Surg Neurol. 2007. 67: 65-73
4. Hu HJ, Liao MY, Xu LY. Clinical utility of dual-energy CT for gout diagnosis. Clin Imaging. 2015. 39: 880-5
5. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T. 2012 American College of Rheumatology guidelines for management of gout Part I: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012. 64: 1431-46
6. Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T. 2012 American College of Rheumatology guidelines for management of gout Part II: Therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012. 64: 1447-61
7. Konatalapalli RM, Demarco PJ, Jelinek JS, Murphey M, Gibson M, Jennings B. Gout in the axial skeleton. J Rheumatol. 2009. 36: 609-13
8. Nicholls A, Snaith M, Scott J. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973. 1: 449-51
9. Qaseem A, Harris R, Forciea M, Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017. 166: 58-68
10. Toprover M, Krasnokutsky S, Pillinger MH. Gout in the spine: Imaging, diagnosis, and outcomes. Curr Rheumatol Rep. 2015. 17: 70