- Department of Neurosurgery, All India Institute of Medical Science, Raebareli, Uttar Pradesh, India.
- Department of Neurosurgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Correspondence Address:
Arun Kumar Srivastava
Department of Neurosurgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
DOI:10.25259/SNI_425_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Suyash Singh1, Arun Kumar Srivastava2, Sanjog Gajbhiye2, Kamlesh Singh Bhaisora2, Awadhesh Kumar Jaiswal2, Sanjay Behari2. Venous corridors in gravity-assisted retractor-less occipito-transtentorial approach – Our experience of an avenue through the tentacles of pod. 18-Nov-2020;11:399
How to cite this URL: Suyash Singh1, Arun Kumar Srivastava2, Sanjog Gajbhiye2, Kamlesh Singh Bhaisora2, Awadhesh Kumar Jaiswal2, Sanjay Behari2. Venous corridors in gravity-assisted retractor-less occipito-transtentorial approach – Our experience of an avenue through the tentacles of pod. 18-Nov-2020;11:399. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10402
Abstract
Background: Occipital transtentorial approach for selected posterior third ventricular or retrosplenium region tumors provides an ergonomic and safe access. Over centuries, the opponents of this approach highlight the problem of postoperative visual field defect, related to the retraction of occipital lobe. The aim was to describe the surgical nuances of gravity-assisted retractor-less occipital-transtentorial approach (GAROTA) as a modification of originally described GAROTA to minimize the complications with a similar ease of surgery.
Methods: In this study, we have retrospectively analyzed our prospectively maintained surgical databases of patients operated by occipito-transtentorial from 2015 to 2019. Demographic variables, preoperative and postoperative neurological deficits (especially visual field defect) were analyzed. Radiological data included relation of veins with tumor, presence of hydrocephalus, size, and extent of lesion.
Results: Fifteen patients underwent GAROTA (right-sided extension, n = 7; left-sided extension, n = 4; and midline lesions, n = 4). Headaches (73.3%) and diplopia (40%) were the most common symptoms. No patient had any postoperative visual deficits in both short-term and long-term follow-up.
Conclusion: A thorough anatomical knowledge of posterior third interhemispheric region in the semi-prone position is required for GAROTA. Meticulous arachnoid dissection around the deep venous complex and release of cerebrospinal fluid through the cisterns is required. Postoperative cortical vision loss may be prevented by following the key surgical principles in GAROTA.
Keywords: Field defect, Interhemispheric, Retraction less, Transtentorial
INTRODUCTION
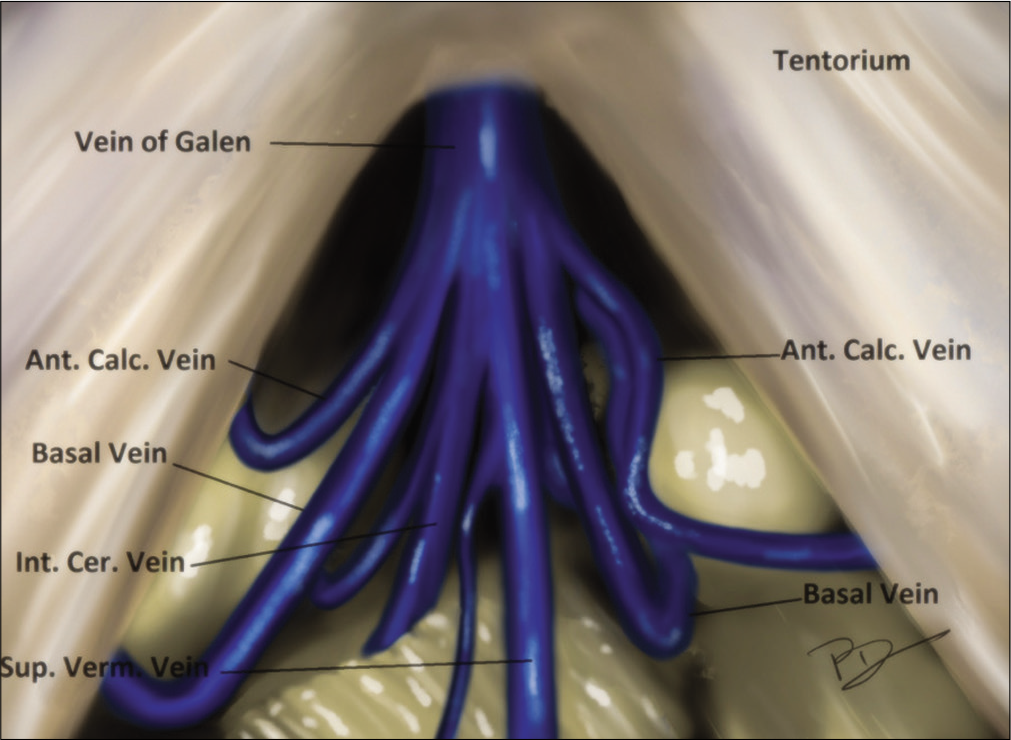
The posterior third ventricular region possesses a perilous challenge due to the presence of enormous deep venous channels and remote access. These tumors occupy a central position that is equidistant from various traditional cranial points. The deep central location is further complicated by the intimate contact with important deep venous system including the vein of Galen, the precentral cerebellar vein, basal vein of Rosenthal (BVR), and the internal cerebral veins (ICVs).[
MATERIALS AND METHODS
Study design
We performed a retrospective analysis of our prospectively maintained surgical databases, from our hospital discharge codes, for all the patients, who underwent surgery for posterior third ventricular region tumor at our institution between 2015 and 2019. Institutional ethical board approval was obtained to review the medical records and neuroimaging studies of these patients. Patient consent is obtained at the time of surgery for the use of imaging as per our departmental protocol. Clinical variables included age and gender at admission, clinical presentation, and any other coexisting medical conditions. We analyzed the pre- and postoperative neurological status from hospital records. Radiological data included relation of veins with tumor, presence of hydrocephalus, size of lesion, and lateral extent of lesion [
Inclusion criteria
Between 2015 and 2019, a total of 69 patients with pineal region/posterior third ventricular tumors were included in the study, of which 57 cases underwent endoscopic transventricular biopsy with or without stereotactic/neuronavigation guidance while 12 cases underwent OTT. Among the 57 patients, where biopsy was performed, 12 patients were offered surgical excision (OTT) and remaining 45 patients were referred to radiotherapy department. Six patients were referred back from radiotherapy department either after recurrence or nonresponsiveness. Therefore, 30 patients underwent surgical excision in this period. Out of these 30 patients, 15 patients were operated by the gravity-assisted retraction-less OTT (GAROT). The pre- and postoperative visual assessment was done by the neuro-ophthalmologist.
Surgical technique
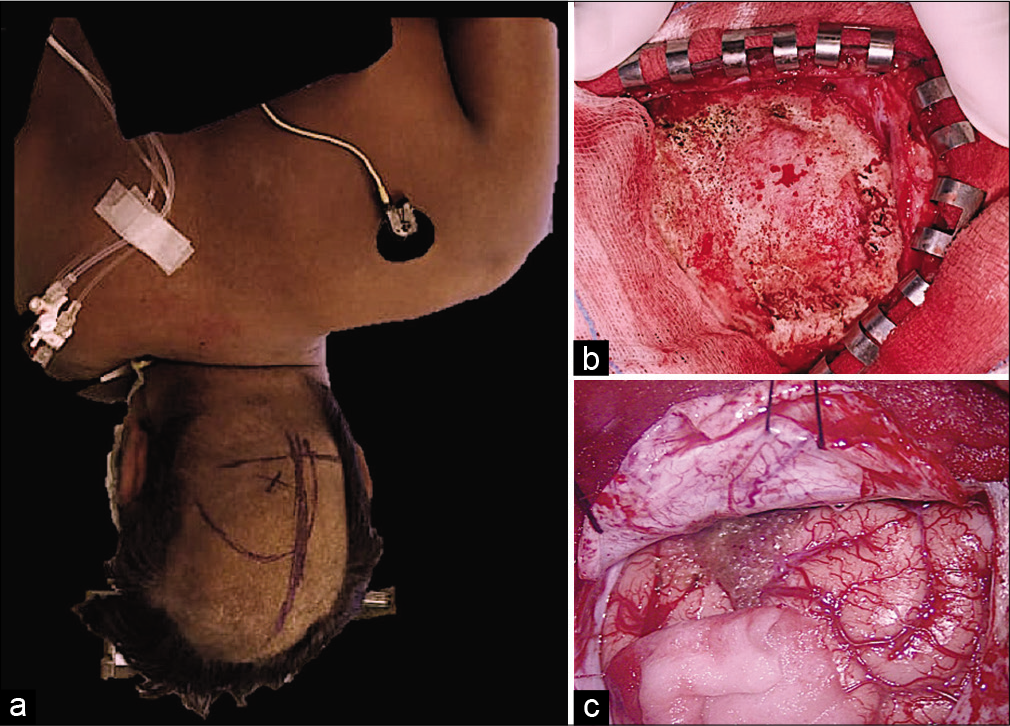
The patient was positioned in the lateral position with the side of pathology facing the floor. The body and head were further rotated 30–45 degrees (chest tilted toward the floor) [
Figure 3:
(a) The patient was positioned in the lateral position with the side of pathology facing the floor. The body and head were further rotated 30–45 degrees; (b) “J”-shaped skin flap was raised just starting below the external occipital protuberance and extending 1 cm below vertex; (c) dura was cut in cruciate manner and hinged toward sagittal sinus and transverse sinus side.
Figure 4:
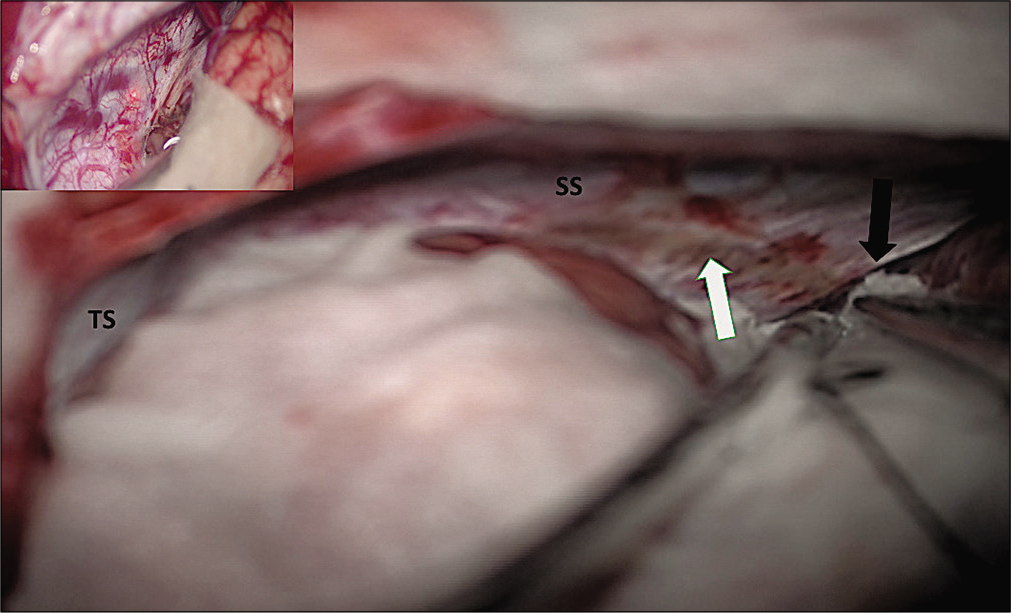
Intraoperative photographs showing posterior interhemispheric corridor on the right side with straight sinus and transverse sinus with and adequate exposure up to free edge of tentorium. Picture-in-picture photo shows straight sinus and transverse sinus. Black arrow shows free edge of tentorium over the ambient cistern and the white arrow shows coagulated tentorium surface.
Within the posterior interhemispheric corridor, the following veins are encountered (a) bridging veins from occipital lobe to SSS and (b) internal occipital vein (IOV) draining into VOG. Both the above veins were preserved as the retraction induced injury was avoided in all the cases.[
The incision on tentorium is calculative and it should be cut only after confirming position of the straight sinus and TS [
Video 1:
Intraoperative video showing the technique of widening the surgical corridor in GAROTA lengthening of draining vein. The tentorium is cut from free margin and the trajectory taken. The up-turned part of needle is used to hinge the dura, which is further coagulated and divided. The confluence of veins in the posterior third ventricular region is surrounded by thich arachnoid web. The arachnoid dissection in the various venous corridors not only eases cerebrospinal fluid decompression but also provides access to tumor. The tumor has thin wall and after aracnoid dissection, one can appreciate the whole lateral wall of tumor.Figure 5:
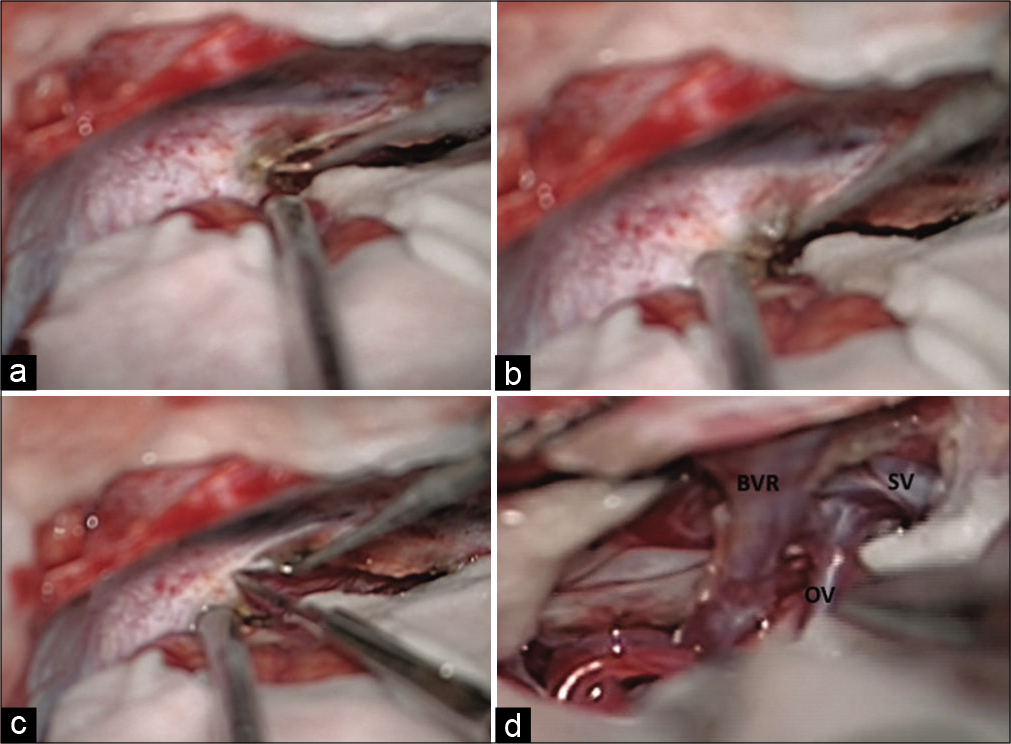
Intraoperative photographs showing steps, technique, and trajectory for cutting the tentorium using up-turned tip of needle and knife. The coagulated tentorium surface is lifted up (a and b) and cut (c). Ambient cistern is opened and venous corridors are defined after arachnoidal dissection (d). BVR: Basal vein of Rosenthal, OV: Occipital vein, SV: Splenial vein.
Ongoing deep, the dense arachnoid of quadrigeminal cistern is seen draping the confluence of venous channels. The veins are encountered posterior to the splenium of corpus callosum which are (a) vein of Galen (VOG), (b) bilateral internal cerebral, (c) splenial veins (SVs), (d) BVR, and (e) vein of cerebellomesencephalic fissure.
Depending on the location of the tumor, the following corridors are proposed through which tumors were approached.
- Infrasplenial
- Occipito-Rosenthal
- Inter-Rosenthal
- Post-Rosenthal.
The main principle of this kind of positioning was to exploit gravity assisting the retraction of the occipital lobe which was further accentuated after release of the CSF from the ambient and quadrigeminal cisterns and finally degree of retraction was achieved through dynamic retraction using the suction tip and the bayonetted instruments (used for dissection mainly surgical bipolar) in the dominant hand.
RESULTS
Fifteen patients underwent surgery between 2015 and 2019. Seven patients had right-sided extension, four had left-sided extension, and four had midline lesions (based on whether lesion was draped between bilateral venous complexes or extending beyond the lateral veins like BVR). There were 10 males and five females, who ranged from 16 to 56 years in age (mean age, 31.2 ± 10.7 years). Two patients underwent resurgery for residual tumor (one was operated by OTT and other were operated by the SCIT approach). These included craniotomies with significant residual tumor which were found to be nonresponsive to chemotherapy with progression of tumor noted on follow-up imaging studies.
Headaches and diplopia were the most frequent presenting symptoms and occurred in 11 patients (73.3%) and 6 patients (40%), respectively. Other presenting symptoms included ataxia in three patients, hemiparesis in two patients, and memory loss in one patient. None of the patient had visual field defect before surgery. It is unusual for posterior third ventricular tumor to present with hemiparesis and both these patients (with preoperative motor deficit) experienced further deterioration. One of them improved to preoperative power in follow-up, while other remained in deteriorated status.
Thirteen patients had lesion centered along the posterior midline and remaining two patients had lesions centered along the posterolateral aspect. All the tumors extended along the rostrocaudal axis of the posterior incisural space along the splenium of the corpus callosum, atrium of the lateral ventricle, and superior colliculus.
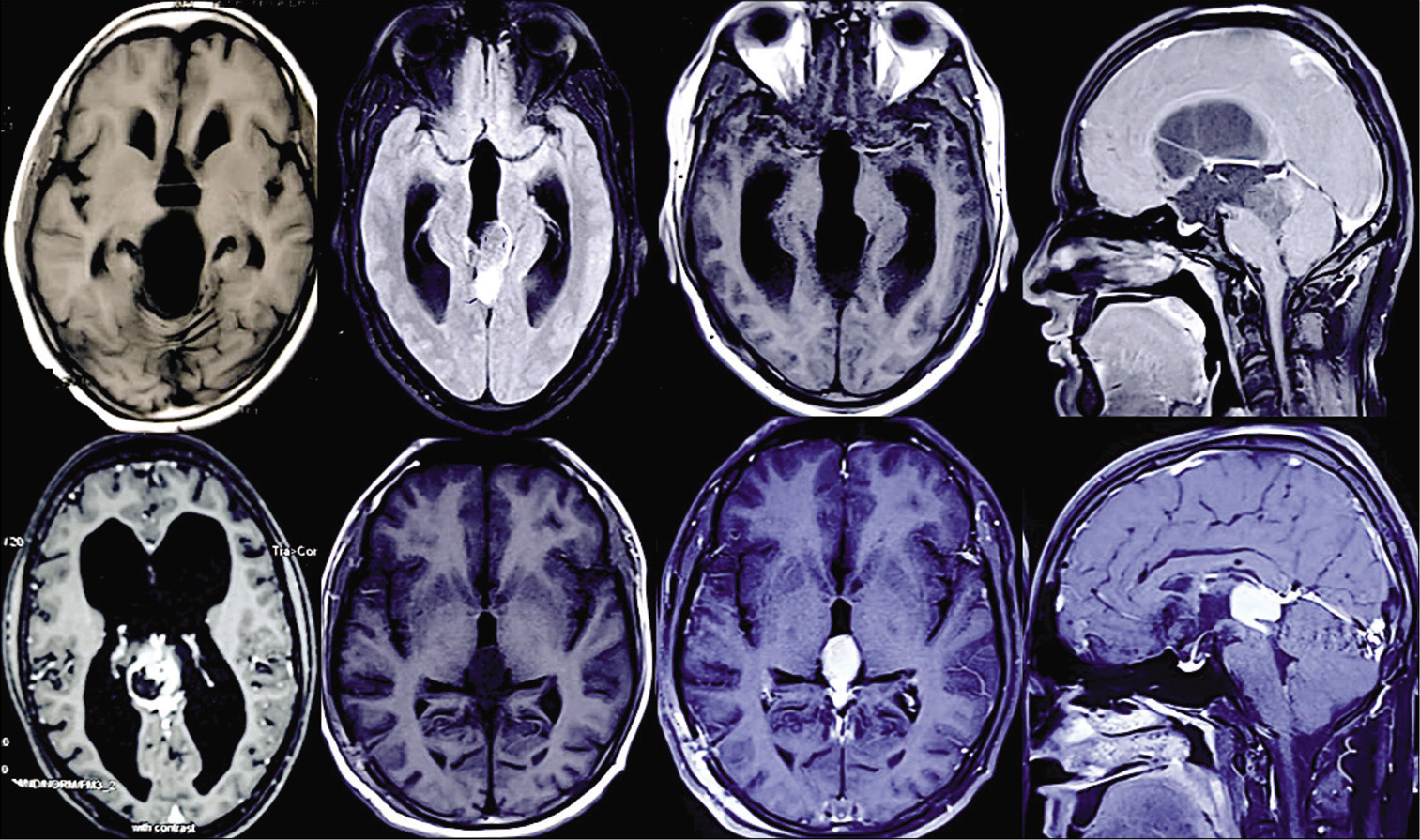
Of the 15 patients reported in this series, 7 had contrast enhancement. There was no relationship noted between the location or extent and postoperative tumor histology or outcome. Hydrocephalus was present in 10 preoperative MRI, out of which, two patients needed ventriculoperitoneal shunt preoperatively. Intraoperative external ventricular drainage was done in 7 patients. One patient required postoperative VP shunt. Shunt malfunction was not encountered in our series.
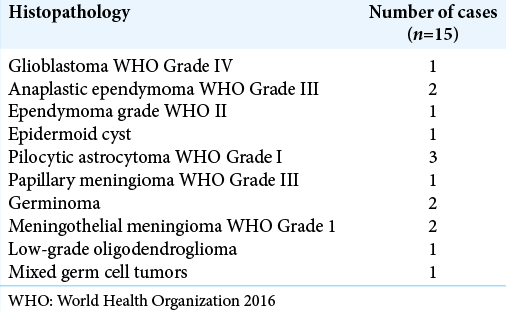
Postoperative postcontrast MRI scans revealed that 11 patients had gross total resections and 4 patients had near- total resections. In these 4 patients with near total, complete tumor removal was not done either because tumor was adhered to the walls of the great VOG or the nearby venous complex. In one patient, the tumor tissue had infiltrated normal brain stem parenchyma and a safe surgical plane between tumor and infiltrated parenchyma was not apparent and while attempting the same, there was significant drop in the SSEP which was immediately restored once tumor was left behind. The histopathological distribution in our series is shown in [
There were no operative deaths in our series. Among the 15 patients, 12 were neurologically intact or had unchanged preexisting neurological deficits at 1 week after surgery. Three patients had new neurological deficits or worsening of preoperative deficits: two patients had hemiparesis and one patient had upgaze paresis.
At the 3-month and 6-month follow-up, 13 patients were neurologically intact. One patient with hemiparesis had improvement in motor power to preoperative status. One patient developed hydrocephalus at 2-month follow-up; he was a 34-year-old male, who presented with vomiting and ataxia, and underwent ventriculoperitoneal shunt. Another patient had persistent hemiparesis.
One patient was previously operated by SCIT approach before and needed resurgery for symptomatic recurrence. This patient had persistent postoperative homonymous hemianopia. Otherwise, no patient in our series had new-onset visual field defect, either in postoperative period or 6-month follow-up.
DISCUSSION
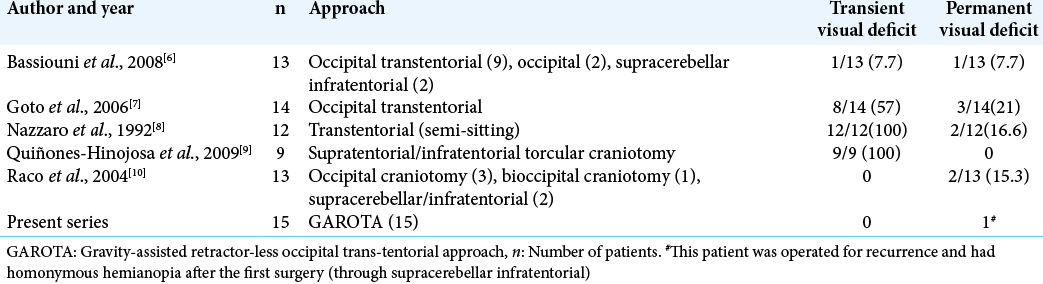
The roof of cerebellomesencephalic fissure is related to posterior incisural space. The contents include (a) VOG, (b) splenium of the corpus callosum, (c) hippocampal commissure, (d) pulvinar of the thalamus, (e) fornix, and (f) parahippocampal gyri. Surgical approach to these deep structures includes OTT and SCIT; the choice of which depends on location and lateral extension of tumor. The OTT approach provides excellent exposure of paramedian lesions of the precentral cerebellar fissure and posterior incisural space. Among all other approaches for this region, OTT provides safest (in terms of cortical damage) corridor, is most surgeon friendly (as far as ergonomics are concern), and provides maximum desirable exposure. The major issue with OTT is retraction of occipital lobe which may lead to postoperative homonymous hemianopia. Various studies have reported transient visual field loss ranging from 19% to 100% of patients and permanent visual field loss in nearly 15–20% of patients [
The complications such as seizures, hemiparesis, and disconnection syndrome are also avoided with GAROT, however, the complex anatomy of dural-falx folds and Galenic system in 90-degree tilted orientation, upsurges technical challenge for surgeon. Similar approaches are described in literature like the posterior interhemispheric parasplenial approach by Yasargil.[
The cadaveric studies showing anatomical structures in sub-splenium region depicts normal architectural pattern of venous complex.[
The venous corridors utilized are as follows:
Infrasplenial corridor between ICV and IOV: This is preferred to see the rostral part of the tumor with an advantage of separating the tumor from the ICV which is pushed superiorly and usually not accessible from behind. A little dynamic traction may be applied on the splenium, pushing it superiorly, to widen the corridor. Sometime it is necessary to open the arachnoid from this corridor, in cases where arachnoid over Galen complex is thick. The SV can be encountered pushed anteriorly and superiorly further creating surgical window between IOV and SV[ Occipito-Rosenthal corridor between IOV and Rosenthal vein: This is utilized to dissect the anterosuperior lateral part of the tumor. We found that it is always better to dissect and coagulate the tumor capsule of the tumor using this corridor without internal decompression, as the natural turgidity of tumor may help in meticulous dissection and coagulation will keep on shrinking the tumor. However, the contralateral dissection should not be attempted without internal decompression of tumor Inter-Rosenthal corridor between the ipsilateral and contralateral RV: This is most commonly entered corridor. Majority of lateral and posterior part of the tumor is exposed though this corridor and we recommend to start the tumor decompression from this corridor whenever possible. The trajectory will automatically lead the surgeon inside posterior part of third ventricle and thereby CSF drainage is facilitated. This corridor further enables the surgeon to access contralateral portion of tumor, without cutting the flax. The arachnoid lengthening of RV further gives scope for dynamic manipulation of vein during the surgery Post-Rosenthal corridor between RV and precerebellar vein (PCV) will expose the caudal most portion of tumor. The PCV may be excised safely to ascertain a better control near the collicular plates.
The exposure through these venous corridors was maximized by meticulous dissection of the arachnoid cuff around the veins draining into the deep venous complex. With advent of neuronavigation system and minimally invasive keyhole approach, few authors propose differential approach according to exact location of tumor. The OTT is, therefore, subdivided as interhemispheric and lateral OT approached and accordingly the tentorium is cut in two different fashions. The interhemispheric corridor provides better exposure of region superior to the corpus callosum and to the atrium of the lateral ventricle compared to the lateral OT approach.[
Importance of neck position in gravity-assisted retractorless occipital trans-tentorial approach (GAROTA)
Flexion/extension: The further decision of working more posterior that is toward collicular plate versus more anterior that is infrasplenial or toward posterior third ventricular area can be comforted by simple modification in neck extension for posterior visibility and neck flexion for anterior visibility.
CONCLUSION
GAROT is safe and effective approach for posterior third ventricle tumors. The principle of GAROTA revolves around the “gravity-assisted retraction” of the occipital lobe. This retraction is further supplemented by the use of dynamic retraction of suction and surgical bipolar/bayonet. A gentle manipulation of neck (flexion/extension) during positioning the patient helps the surgeon to access to the desired region of the posterior third ventricle area. The presence of cortical bridging occipital veins, posterior to the lambdoid suture necessitates dynamic retraction for an optimal surgical corridor. The tentacles of pods (venous anatomy) are naturally pushed apart to provide few corridors and facilitate the tumor excision. These corridors are infrasplenial corridor between ICV and IOV, occipito-Rosenthal corridor between IOV and Rosenthal vein, inter-Rosenthal corridor between the ipsilateral and contralateral RV, and post-Rosenthal corridor between RV and PCV. The chances of postoperative visual field defect are negligible in GAROTA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Bassiouni H, Asgari S, König HJ, Stolke D. Meningiomas of the falcotentorial junction: Selection of the surgical approach according to the tumor type. Surg Neurol. 2008. 69: 339-49
2. Behari S, Garg P, Jaiswal S, Nair A, Naval R, Jaiswal AK. Major surgical approaches to the posterior third ventricular region: A pictorial review. J Pediatr Neurosci. 2010. 5: 97-101
3. Chi JH, Lawton MT. Posterior interhemispheric approach: Surgical technique, application to vascular lesions, and benefits of gravity retraction. Neurosurgery. 2006. 59: ONS41-9
4. Cohen-Cohen S, Cohen-Gadol AA, Gomez-Amador JL, AlvesBelo JT, Shah KJ, Fernandez-Miranda JC. Supracerebellar infratentorial and occipital transtentorial approaches to the pulvinar: Ipsilateral versus contralateral corridors. Oper Neurosurg (Hagerstown). 2019. 16: 351-9
5. Goto T, Ohata K, Morino M, Takami T, Tsuyuguchi N, Nishio A. Falcotentorial meningioma: Surgical outcome in 14 patients. J Neurosurg. 2006. 104: 47-53
6. Jameson K. Excision of pineal tumors. J Neurosurg. 1971. 35: 550-3
7. Lopez-Gonzalez MA, Jaeger A, Kaplan B, Eastin TM, Kore L, Gospodarev V. Retractorless interhemispheric transtentorial approach for large lesions in the posterior incisural space. Surg Neurol Int. 2019. 10: 130
8. Ma Y, Lan Q. An anatomic study of the occipital transtentorial keyhole approach. World Neurosurg. 2013. 80: 183-9
9. Matsuo S, Baydin S, Güngör A, Middlebrooks EH, Komune N, Iihara K. Prevention of postoperative visual field defect after the occipital transtentorial approach: Anatomical study. J Neurosurg. 2018. 129: 188-97
10. Moshel YA, Parker EC, Kelly PJ. Occipital transtentorial approach to the precentral cerebellar fissure and posterior incisural space. Neurosurgery. 2009. 65: 554-64
11. Nazzaro JM, Shults WT, Neuwelt EA. Neuro-ophthalmological function of patients with pineal region tumors approached transtentorially in the semisitting position. J Neurosurg. 1992. 76: 746-51
12. Poppen JL. The right occipital approach to a pinealoma. J Neurosurg. 1966. 25: 706-10
13. Quiñones-Hinojosa A, Chang EF, Chaichana KL, McDermott MW. Surgical considerations in the management of falcotentorial meningiomas: Advantages of the bilateral occipital transtentorial/transfalcine craniotomy for large tumors. Neurosurgery. 2009. 64: 260-8
14. Raco A, Agrillo A, Ruggeri A, Gagliardi FM, Cantore G. Surgical options in the management of falcotentorial meningiomas: Report of 13 cases. Surg Neurol. 2004. 61: 157-64
15. Rhoton AL Jr. The cerebral veins. Neurosurgery. 2002. 51: S159-205
16. Yamamoto I. Pineal region tumor: Surgical anatomy and approach. J Neurooncol. 2001. 54: 263-75
17. Yasargil MG.editors. Microneurosurgery. Stuttgart: Thieme Verlag; 1996. 4B: 35-64






James Ausman
Posted November 25, 2020, 9:29 am
To the Authors: There is a reference you should include in your bibliography on this subject.
Published in Surgical Neurology International By Ausman et al 1988, 29: 298-306 (Three-Quarter Prone Approach to the Pineal-Tentorial Region) that discusses all the reasons for adopting this approach and how the patient should be positioned.