- Department of Neurosurgery, Northeast National Medical Center, Monterrey, Nuevo Leon, Mexico,
- Department of Epilepsy, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
- Department of Neurocysticercosis, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
Correspondence Address:
J Javier Cuellar-Hernandez
Department of Neurocysticercosis, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
DOI:10.25259/SNI_755_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: J Javier Cuellar-Hernandez1, Alan Valadez-Rodriguez1, Ramon Olivas-Campos1, Paulo Tabera-Tarello1, Daniel San Juan-Orta2, Roberto Segura-López1, Agnès Fleury3. Intrasellar cysticercosis cyst treated with a transciliary supraorbital keyhole approach – A case report. 11-Dec-2020;11:436
How to cite this URL: J Javier Cuellar-Hernandez1, Alan Valadez-Rodriguez1, Ramon Olivas-Campos1, Paulo Tabera-Tarello1, Daniel San Juan-Orta2, Roberto Segura-López1, Agnès Fleury3. Intrasellar cysticercosis cyst treated with a transciliary supraorbital keyhole approach – A case report. 11-Dec-2020;11:436. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10440
Abstract
Background: Neurocysticercosis is the most common parasitic disease affecting the central nervous system. Isolated sellar cysticercosis cysts are rare and can mimic other sellar lesion as cystic pituitary adenoma, arachnoid cyst, Rathke cleft cyst, or craniopharyngioma. The surgical resection is mandatory because the cysticidal drugs are ineffective, however, new microsurgical approaches are emerging to reduce complications and need to test in this condition. We present a patient with a sellar cysticercosis cyst treated by transciliar supraorbital keyhole approach.
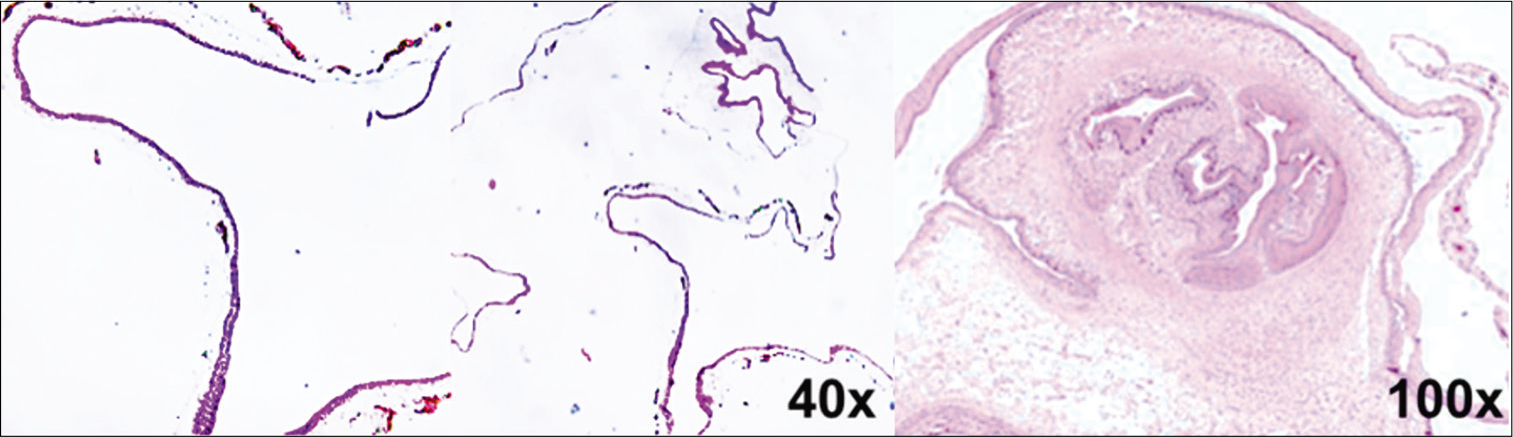
Case Description: A 45-year-old female with presented with chronic severe headaches, progressive deterioration of 6 months in visual acuity and bitemporal hemianopia. The pituitary hormonal levels were normal. Magnetic resonance findings showed a sellar and suprasellar cyst and underwent a microsurgical supraorbital transciliar keyhole approach for lesion resection. Pathologically, the lesion demonstrated a parasitic wall characterized by wavy, dense cuticle, and focal globular structure, surrounding inflammatory reaction with plasma cells. Postoperatively, the patient recovery fully neurologically.
Conclusion: Intrasellar cysticercosis cyst causes significant neurological deficits due to its proximity to the chiasm, optic nerves, pituitary stalk, and the pituitary gland. Surgical section is an effective treatment. The supraorbital keyhole craniotomy offers satisfactory exposure, possibility of total resection with dissection of the supra and parasellar structures, short operative time, less blood loss, short hospital stay, and good overall surgical outcome.
Keywords: Cysticercosis cyst, Keyhole, Sellar, Supraorbital craniotomy
INTRODUCTION
Neurocysticercosis (NCC) is the most common parasitic disease affecting the central nervous system (CNS), cysticercosis can arise almost anywhere in the CNS (e.g., intraparenchymal, cisternal, or intraventricular) and in rare cases has been described in the sellar or suprasellar region.[
In our country, where NCC is endemic, there are scare reports since 1985[
CASE PRESENTATION
A 45-year-old woman living in an endemic area for cysticercosis, presented with chronic severe headaches, progressive deterioration of 6 months in visual acuity with blurring of her left-sided vision that caused her to bump into objects on her left side and later in the right eye.
On neurological examination, visual acuity in the right eye 20/60 and in the left eye 20/80, isochoric pupils of 2 mm and normal light reflex, bitemporal hemianopia, and extraocular movements were of full range, fundus examination was within normal limits.
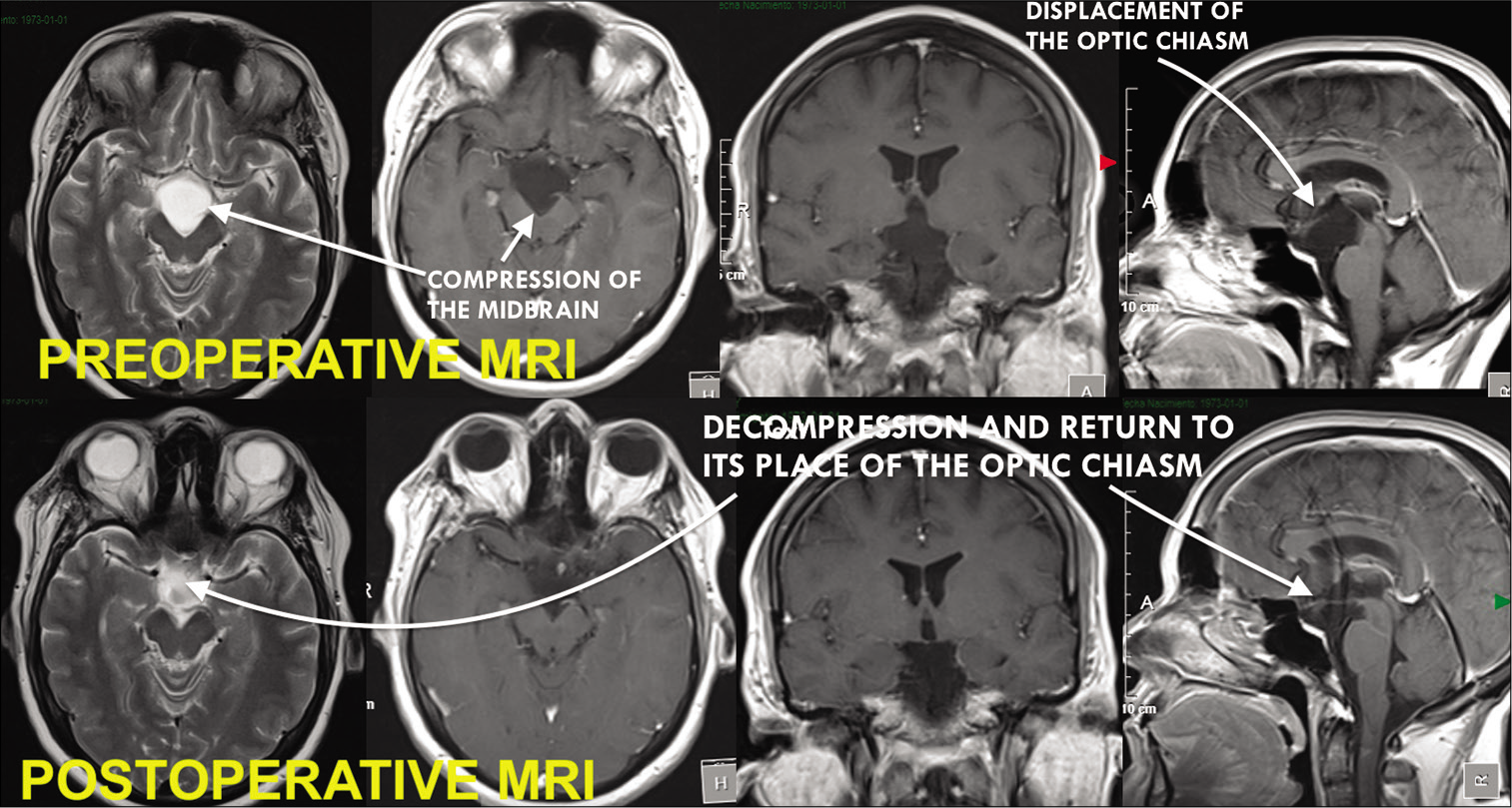
Pituitary hormonal assay was carried out and was found to be normal. Brain magnetic resonance (MR) showed a cystic image in the sellar and suprasellar region (i.e., 17 × 20 × 22 mm) hyperintense on T2 sequence and hypointense on T1 with no capsule enhancement on the contrast study, causing upward displacement and compression of the optic chiasm [
Figure 1:
Preoperative magnetic resonance imaging (MRI) showed cystic image in the sellar and suprasellar region (i.e., 17 × 20 × 22 mm) hyperintense on T2 sequence and hypointense on T1 with no capsule enhancement on the contrast study, causing upward displacement and compression of the optic chiasm. Postoperative MRI reveals resection of the cyst with liberation of the sellar and interpeduncular arachnoid space.
Surgery
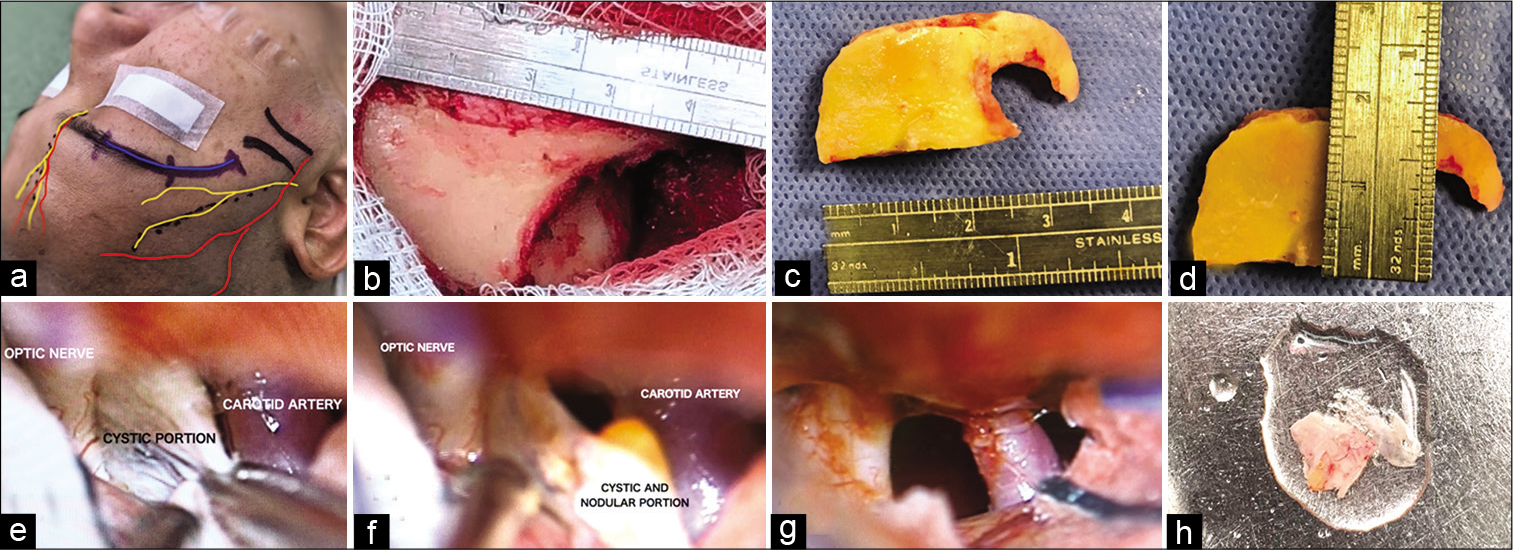
The patient underwent a supraorbital transciliar keyhole approach. This was performed utilizing microscopic visualization and we identified a cyst with a firm avascular capsule, clear liquid content, with lax adhesions to the chiasm, optic nerves and to a small area of thickened arachnoid and diaphragm sellae, without arterial compromise that was fully resected without complications or incidents [
Figure 2:
(a) Planning the approach taking into account the anatomical landmarks (supraorbital nerve and artery, frontal branch of the facial nerve, zygomatic arch, and supraorbital notch). (b-d) Single frontobasal burr hole was made posterior to the temporal line, thereafter, a “c”-shaped line is cut from the medial border of the frontobasal cut, thus creating a bone flap with a width of 2 × 3 cm. (e) Surgical field where is observed the internal carotid artery, optic nerve, and the cyst. (f and h) Once the cystic portion was resected, we identified a nodular portion compatible with the scolex. (g) Free carotid optic space due to total cyst resection.
DISCUSSION
NCC is the larval phase of Taenia solium in the human CNS, being this the most common parasitic brain disease in the world.[
Being divided into parenchymal and extraparenchymal disease, the location of the parasite defines the pathophysiology, clinical, therapeutic, and prognostic outcomes of this illness. This difference is important for the effectiveness of the cysticidal treatment, having a very poor effectiveness in cysts located in the basal cisterns and the intraventricular space, because of the lack of interaction with the immune cells and a very low concentration of cysticidal drugs in this compartment.[
The extraparenchymal form that accounts for 15–30% of the cases has variable clinical manifestations including intracranial hypertension, cranial nerve abnormalities, and hydrocephalus.[
The use of computed tomography (CT) and MR imaging helps making diagnosis of sellar pathologies including pituitary adenomas, arachnoid cysts, Rathke cleft cysts, craniopharyngioma, and NCC. The appearance of bony expansion and erosion of the sella with a hypodense cystic lesion, and occasional calcification mass, there, or in other brain region, supports the diagnosis of sellar NCC. On MR, this disease presents with a cyst that have low or high T1 signal and a heterogeneous content, also presenting enhancement and thickening of the infundibulum, and leptomeningeal enhancement in the parasellar region.[
The medical treatment in sellar NCC disease is not well established. The use of antiparasitic drug preoperatively in surgery candidate patients can result in disruption of the parasite integrity and inflammatory response that can make more difficult the cyst removal. For this reason, the surgical removal of the cysticerci recommended rather than medical therapy[
The supraorbital approach allows a corridor to much of the anterior and middle cranial base and minimizes or eliminates frontal lobe retraction while exposing lesions of the sella, sphenoid wing, posterior clinoid, and other regions of the skull base. Neurovascular structures are also well visualized, and the cosmetic result is optimal.[
The case we present had a campimetric deficit as the main clinical presentation caused by chiasmatic compression secondary to an intrasellar cyst. This clinical manifestation has been reported in the revised bibliography in very few cases,[
CONCLUSION
Intrasellar cysticercosis cyst causes significant neurological deficits due to its proximity to the chiasm, optic nerves, pituitary stalk, and the pituitary gland. Surgical section is an effective treatment for the mass effect and performing it through minimally invasive surgery improves the outcome of the patient. The supraorbital keyhole craniotomy offers satisfactory exposure, possibility of total resection with dissection of the supra and parasellar structures, short operative time, less blood loss, short hospital stay, and good overall surgical outcome.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Carpio A, Fleury A, Hauser WA. Neurocysticercosis: Five new things. Neurol Clin Pract. 2013. 3: 118-25
2. Cheng CM, Noguchi A, Dogan A, Anderson GJ, Hsu FP, McMenomey SO. Quantitative verification of the keyhole concept: A comparison of area of exposure in the parasellar region via supraorbital keyhole, frontotemporal pterional, and supraorbital approaches. J Neurosurg. 2013. 118: 264-9
3. Cheong JH, Kim JM, Kim CH. Neurocysticercosis involving the pituitary stalk: Case report and literature review. J Korean Neurosurg Soc. 2010. 48: 91-3
4. Degiorgio CM, Houston I, Oviedo S, Sorvillo F. Deaths associated with cysticercosis. Report of three cases and review of the literature. Neurosurg Focus. 2002. 12: e2
5. del Brutto OH, del Brutto VJ. Intrasellar cysticercosis: A systematic review. Acta Neurol Belg. 2013. 113: 225-7
6. del Brutto OH, Guevara J, Sotelo J. Intrasellar cysticercosis. J Neurosurg. 1988. 69: 58-60
7. Dutta D, Kumar M, Ghosh S, Mukhopadhyay S, Chowdhury S. Pituitary hormone deficiency due to racemose neurocysticercosis. Lancet Diabetes Endocrinol. 2013. 1: e13
8. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: A focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011. 9: 123-33
9. Ghosh S, Al-Khalili R, Liu JK, Slasky SE. Paradoxical migrating cyst: An unusual presentation of intraventricular neurocysticercosis with a coincidental pituitary adenoma. J Clin Neurosci. 2014. 21: 1066-8
10. Jane JA, Park TS, Pobereskin LH, Winn HR, Butler AB. The supraorbital approach: Technical note. Neurosurgery. 1982. 11: 537-42
11. Jensen TO, Post JJ. Intraventricular neurocysticercosis: Presentation, diagnosis and management. Asian Pac J Trop Med. 2016. 9: 815-8
12. Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S. Natural history of treated subarachnoid neurocysticercosis. Am J Trop Med Hyg. 2020. 102: 78-89
13. Paik KK, Lim SK, Lee HC, Lee EJ, Huh KB, Kim DI. Empty sella syndrome associated with central nervous system cysticercosis. Korean J Intern Med. 1988. 3: 128-31
14. Rafael H, Gomez-Llata S. Intrasellar cysticercosis. Case report. J Neurosurg. 1985. 63: 975-6
15. Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005. 57: 242-55
16. Sibat HF, Valdés LF.editors. Uncommon clinical manifestations of cysticercosis. Novel Aspects on Cysticercosis and Neurocysticercosis. Rijeka, Croatia: InTech; 2013. 8: 199-233
17. Simão D, Teixeira JC, Campos AR, Coiteiro D, Santos MM. Fourth ventricle neurocysticercosis: A case report. Surg Neurol Int. 2018. 9: 201
18. Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015. 12: e1001920
19. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH). Clin Infect Dis. 2018. 66: e49-75
20. Zada G, Lopes MB, Mukundan S, Laws E.editors. Neurocysticercosis of the sellar region. Atlas of Sellar and Parasellar Lesions. Berlin: Springer International Publishing; 2016. p. 419-22