- Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States,
- Department of Neurosurgery, University of Southern California, Los Angeles, California, United States,
- Center for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Randwick, New South Wales, Australia.
Correspondence Address:
Christen M. O’Neal
Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States,
DOI:10.25259/SNI_628_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christen M. O’Neal1, Tressie M. Stephens1, Robert G. Briggs2, Michael E. Sughrue3, Andrew K. Conner1. Navigated transcranial magnetic stimulation following awake craniotomy for resection of glioma: Description of two cases. 11-Dec-2020;11:433
How to cite this URL: Christen M. O’Neal1, Tressie M. Stephens1, Robert G. Briggs2, Michael E. Sughrue3, Andrew K. Conner1. Navigated transcranial magnetic stimulation following awake craniotomy for resection of glioma: Description of two cases. 11-Dec-2020;11:433. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10443
Abstract
Background: Although transcranial magnetic stimulation (TMS) has been indicated as a potential therapy for several neurologic conditions, there is little known regarding its use during the postoperative rehabilitation period in patients with brain tumors. Furthermore, seizures, a common presentation in these patients, are regarded as a major contraindication for TMS therapy.
Case Description: We demonstrate that postoperative continuous theta burst stimulation (cTBS), a patterned form of repetitive TMS, was safely tolerated in addition to current neurorehabilitation techniques in two brain tumor patients, including one patient with a history of tumor-related epilepsy. We administered navigated 5 Hz cTBS to two patients within 48 h following awake craniotomy for tumor resection. Active motor thresholds were measured in both patients before TBS administration to determine stimulus intensity. We used resting-state fMRI to identify likely damaged networks based on postoperative deficits. This aided in TMS planning and allowed deficit targeted therapy contralateral to the lesioned network node. Both patients tolerated TBS therapy well and had no adverse effects, including posttreatment seizures, despite one patient having a history of tumor-related epilepsy.
Conclusion: TBS may be safe in the immediate postoperative period for patients following brain tumor resection. Additional studies are needed to quantify the efficacy of TMS in improving neurologic deficits following tumor resection.
Keywords: Brain tumor, Neurorehabilitation, Postoperative rehabilitation, Seizures, Theta burst stimulation, Transcranial magnetic stimulation
INTRODUCTION
Theta burst stimulation (TBS) is a patterned form of repetitive transcranial magnetic stimulation (rTMS) that utilizes lower frequencies and shorter pulse durations than conventional rTMS.[
Patients with seizures secondary to glioblastoma (GBM) may benefit from postoperative TBS treatment for rehabilitation of neurologic deficits once the tumor, and consequently, the epileptogenic focus has been resected. Of significance, recent literature has established that early neurorehabilitation in acute stroke patients results in improved outcomes, possibly due to the effects on neuroplasticity.[
Despite these potential benefits, there are three main concerns with initiating TBS-based neurorehabilitation in patients with brain tumors. First, these patients often present with seizures,[
In these two patients, we demonstrate that TBS treatment was safely tolerated immediately following awake craniotomy for tumor resection, even in a patient with a medical history of seizures. We also demonstrate that it is possible to find active motor thresholds (aMT) in patients within 48 h after surgery, despite one patient presenting with baseline motor deficits in the contralateral limb.
CASE PRESENTATIONS
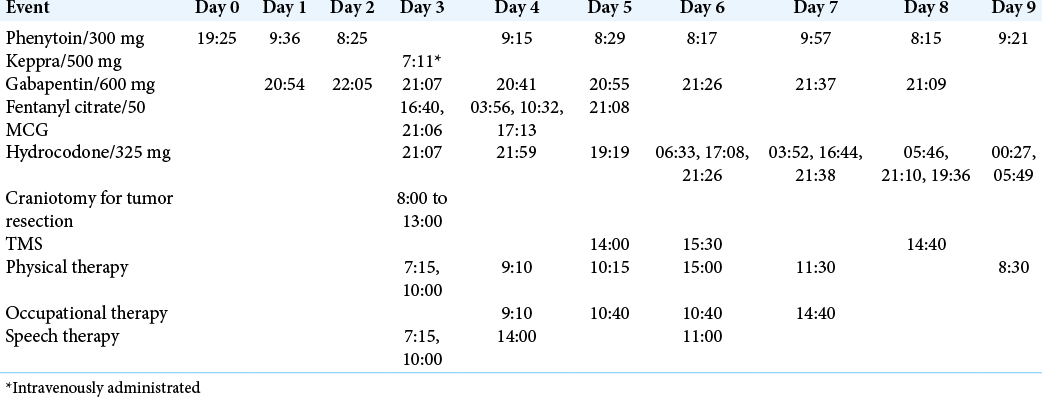
This report is a descriptive series of two cases which analyzed patient outcomes in the context of a new postoperative intervention with cTBS. Informed consent was obtained from both patients to receive the cTBS as an off-label use of the MagVenture MagPro 100× machine. All risks were disclosed and oral and written consent were obtained postoperatively from both patients and their power of attorneys, respectively. Both patients received any indicated physical therapy and speech therapy as part of standard postoperative care.
We performed a retrospective review of both patients described in this manuscript with approval from our Institutional Review Board 3199. We describe all pertinent pre- and postoperative events for both patients based on data obtained from the electronic medical record. Tumor volumes were calculated using preoperative, contrast-enhanced T1-weighted MRIs as described in other studies.[
Patient 1
A 26-year-old right-handed female with an anaplastic astrocytoma of the left insula presented with the right pronator drift [
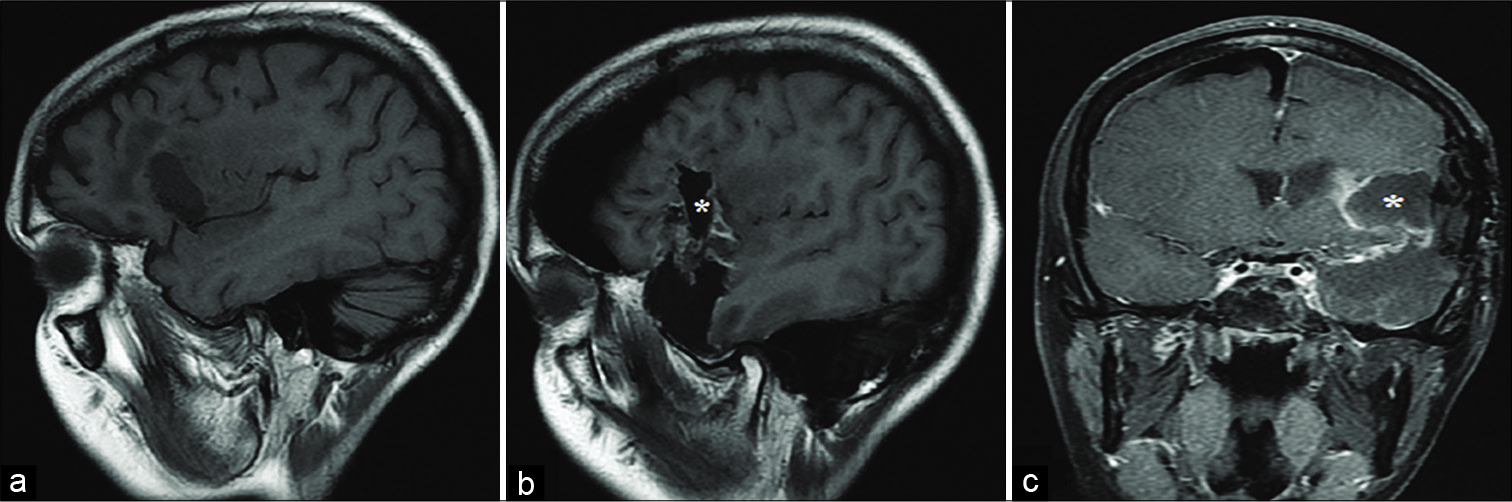
Figure 1:
Pre- and postoperative magnetic resonance imaging of the first patient’s recurrent left insular glioma, glioma resection, and temporal lobectomy. (a) Noncontrast, T1-weighted sagittal image highlights the patient’s previous resection cavity. (b) Sagittal and (c) coronal MR images demonstrate the patient’s postoperative resection cavity as well as resection of the left anterior temporal lobe. *Indicates the postoperative resection cavity in all panels.
Patient 2
A 64-year-old right-handed male with the left temporal GBM presented to neurosurgery with expressive aphasia [
Figure 2:
Pre- and post-operative magnetic resonance imaging of the first patient’s recurrent left insular glioma, glioma resection, and temporal lobectomy. (a) Non-contrast, T1-weighted sagittal image highlights the patient’s previous resection cavity. (b) Sagittal and (c) coronal MR images demonstrate the patient’s post-operative resection cavity as well as resection of the left anterior temporal lobe. ((*) indicates the post-operative resection cavity in all panels.)
Independent component analysis (ICA) and identification of cortical networks
Postoperative rfMRI was obtained in both patients to identify networks through ICA and to target cTBS therapy contralateral to the network nodes associated with the most significant postoperative deficit. The resting-state functional data were preprocessed using the Multivariate Exploratory Linear Decomposition into Independent Components application of the FSL toolbox v. 5.0 (
Treatment rationale
For patient 1, we targeted the motor network through right primary motor cortex (M1) with one session of cTBS.[
Patient 2 demonstrated worsening expressive aphasia postoperatively. Therefore, we attempted to target the language network by stimulating the right inferior frontal gyrus (IFG). The overall goal of this approach was to restore language network function by reducing cortical excitability of the contralesional IFG, thereby reducing severity of the expressive aphasia deficit.[
Stimulation protocol
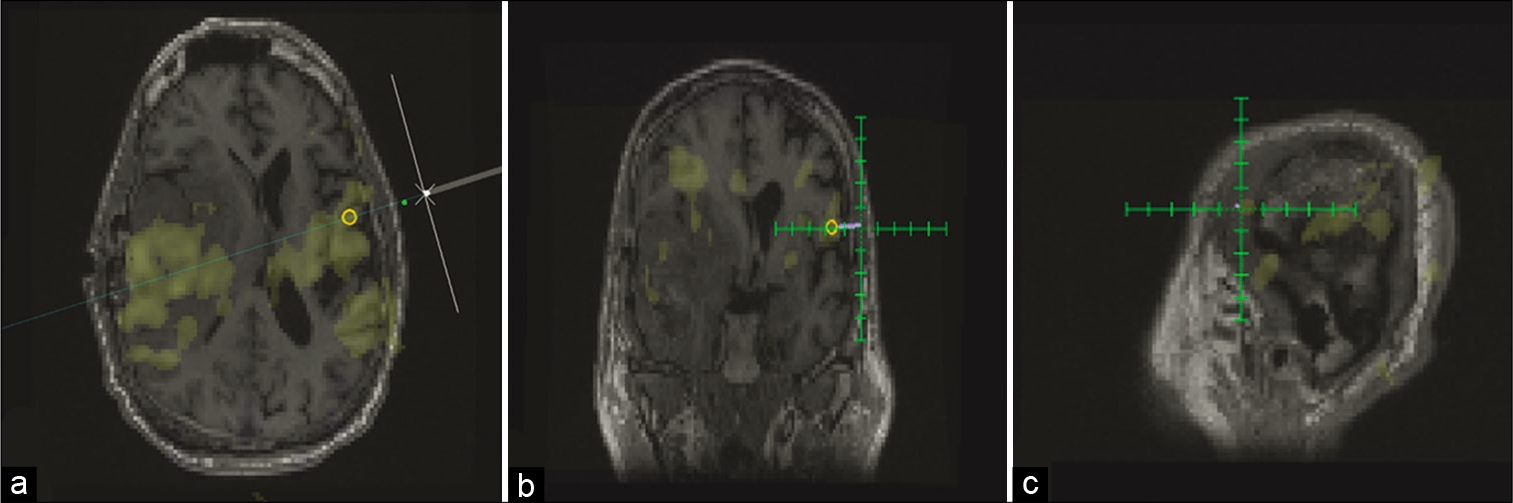
Independent components were loaded into the TMS platform (Magventure MagPro 100×). Each patient’s anatomic image was uploaded as a NIFTI file and then the ICA map at threshold was overlaid to facilitate navigation of the TMS coil to the targeted network node. TMS planning on Localite TMS Navigator Version 3.0.48 (Localite TMS Navigator, Localite, Sankt Augustin, Germany) was initiated, and both the target and entry point were selected to allow guidance of the TMS coil and direction of the current [
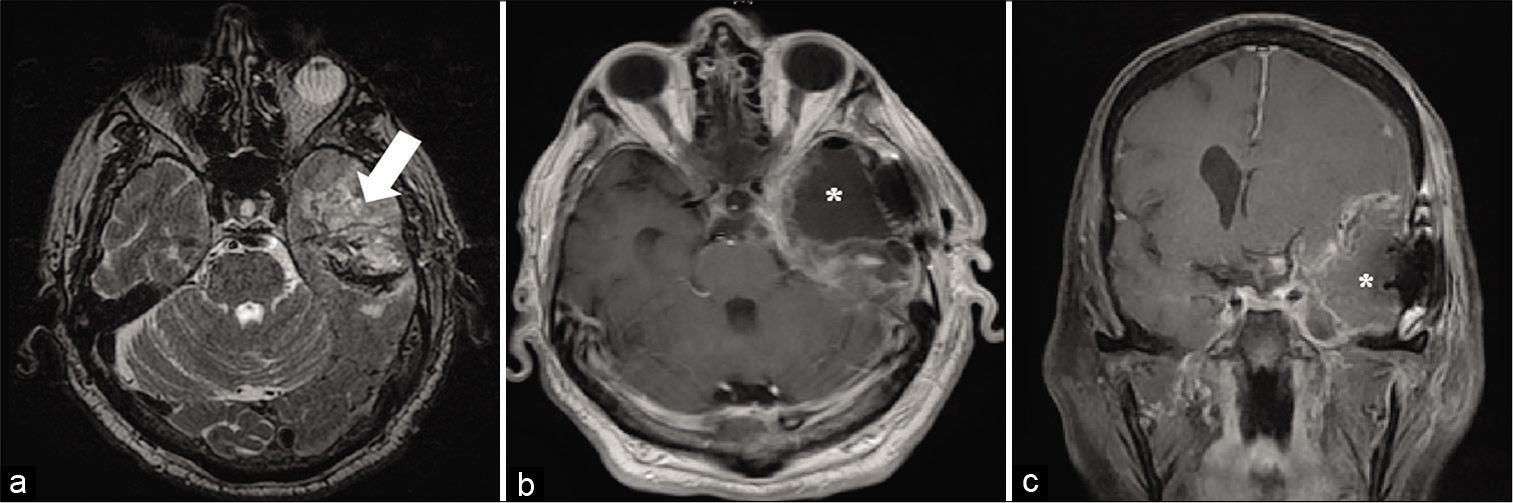
Figure 3:
Pre- and post-operative magnetic resonance imaging of the second patient’s left temporal glioblastoma and its resection cavity. (a) T2-weighted axial image demonstrates the patient’s tumor (white arrow). (b) Axial and (c) coronal MR images demonstrate the patient’s postoperative resection cavity consistent with a left anterior temporal lobectomy. ((*) indicates the post-operative resection cavity in all panels).
Navigated cTBS was administered with a MagVenture MagPro device using a Figure 8 coil at bedside in the ICU for both patients. Patient 1 received one session of 5 Hz cTBS targeted to right M1 at 80% of aMT for a total of 200 pulses. This intervention was administered about 28 h following surgery. Patient 2 received three sessions of 5 Hz cTBS to right IFG at 80% of aMT for a total of 600 pulses, 48 h postoperatively.
RESULTS
Safety of cTBS intervention
During treatment, there were no adverse effects such as itching, tingling, burning, headache, or dizziness, and no serious adverse effects such as seizure. There were no significant changes in vitals following TMS administration.
Clinical assessment – Patient 1
The total length of stay for the first patient was 3 days. Postoperative deficits included continued right hemiparesis graded at 4/5, mild right pronator drift, delayed coordination, and impaired attention, with new deficits including decreased strength of the left lower extremity graded at 4/5 with break-away weakness to resistance, right facial droop, and blurred vision in the left eye. Before discharge, the patient was able to ambulate independently, with lower extremity strength improved to 4+/5 bilaterally. All other postoperative deficits were unchanged at discharge and there were no differences in neurologic examination before and after cTBS administration. At the 4-week follow-up, the left lower extremity strength improved to 5/5 and right upper extremity strength improved to 4+/5. The patient denied any seizures and all postoperative deficits were unchanged. After the 4-week follow-up, patient 1 transferred care to an oncologist closer to her home, though clinic and radiation treatment notes were followed through fax for 4 months postoperatively. Two months postoperatively, this patient continued to have right facial weakness right upper extremity weakness graded at 3/5. Unfortunately, radiation was not initiated for more than 3 months following tumor resection and no chemotherapy was reported. As part of an effort to account for patients lost to follow up, obituaries were reviewed to determine if a patient had died. Through examining obituaries in this patient’s home town, we learned that she died abruptly at 4 months and 2 weeks following surgery, despite having no complications to her postoperative treatment regimen. It is unclear what led to the delay in adjunct treatment for this patient, but partial brain radiation was completed 9 days before death. Although no immediate postradiation complications were reported, the authors believe that the effect of postradiation edema in addition to the treatment delay may have contributed to this patient’s unexpected deterioration.
Clinical assessment – Patient 2
The total length of stay for the second patient was 9 days, however, surgical resection was not performed until hospital day 4. Postoperative deficits included continued mild right pronator drift and apraxia, worsening of expressive aphasia, a change from subjective left lower extremity weakness to 4/5 strength, and new postoperative right dysmetria. At discharge, coordination of the upper extremities had improved with resolution of the postoperative right dysmetria. All other postoperative deficits were unchanged at discharge and there were no differences in neurologic examination before and after cTBS administration. At the 2-week follow-up, the patient was reported to have continued difficulty with word finding, though this was improved from discharge. No other deficits were noted at this follow-up. This patient’s follow-up was limited past 2 weeks, and chemotherapy and radiation were not completed postoperatively. Through examining obituaries in this patient’s home town, we learned that he died 4 months after his surgery. The authors believe that the combination of this patient’s complex presentation with the lack of adjuvant treatment following the second surgical resection likely led to this patient’s rapid deterioration.
Postoperative motor threshold
The aMT for patient 1 was found at 45% maximum stimulator output (MSO), 28 h following awake craniotomy for anaplastic astrocytoma resection of the left insula. The aMT for patient 2 was found at 48% MSO, 48 h following awake craniotomy for residual GBM of the left temporal lobe. Two more sessions of cTBS were administered, one on postoperative day 3 and one on postoperative day 5. The aMT for patient 2 on postoperative day 3 was found at 40% MSO and 38% MSO on postoperative day 5.
DISCUSSION
At present, there is little to no literature on the short-term effects or safety, let alone efficacy, of postoperative TMS as an adjunct to neurorehabilitation in brain tumor patients. We demonstrate two cases of cTBS administered within 48 h of awake craniotomy for tumor resection, with no immediate postoperative complications or adverse effects to TBS or at the 2-week follow-up. These preliminary results suggest that postoperative TBS is potentially safe and well-tolerated during the postoperative recovery period.
While this case series adds to the existing literature on TBS as a tool for motor rehabilitation, to the author’s knowledge, it is the first description of postoperative TBS for aphasia in glioma patients. The TBS protocol for patient 2 was similar to protocols utilized in poststroke patients with residual aphasia.[
Although this study is limited by a number of factors including very short-term follow-up in only two patients, it demonstrates that postoperative cTBS was administered safely in the two patients presented, as well as possibly aiding in transient improvement of neurological deficits. In one study, TBS has been linked to treatment-induced seizures in patients without a prior history of seizures, while other rTMS stimulation protocols have been associated with a limited incidence of seizures.[
While we demonstrate that postoperative TBS was safely administered to two immediate postoperative awake craniotomy patients, further examination of safety concerns in brain tumor patients following tumor resection is warranted. However, this study is not without its limitations. The accuracy of the fMRI postoperatively may have been compromised due to surgically induced signal changes. In attempt to minimize these changes, both scans were acquired within 24 h of surgery. Both patients had a limited survival following their tumor resection, with patient 1 having an overall survival of 6 years and patient 2 having an overall survival of 4 months. The lack of follow-up with prompt adjuvant therapies in both patients, in addition to the recurrent nature of patient 1’s tumor and patient 2’s complex presentation with tumor hemorrhage, likely complicated the disease course for patients. While we believe combined TBS and neurorehabilitation following craniotomy for tumor resection shows promise for improving postsurgical patient outcomes, further examination of the efficacy in brain tumor patients is necessary. Randomized controlled trials comparing postoperative outcomes of patients receiving TBS and neurorehabilitation, as opposed to neurorehabilitation alone, will be critical in understanding the lasting effects of TBS in brain tumor patients. Future research should be aimed at determining the efficacy and long-term effects of TBS as an adjunct to traditional postoperative neurorehabilitation.
CONCLUSION
We present two cases of patients receiving a postoperative TBS and neurorehabilitation intervention with no adverse effects. Although one patient had a history of tumor associated seizures, both patients tolerated TBS treatment well and experienced no adverse effects. We show that TBS was safely undertaken, though the efficacy and long-term effects of TBS as a postoperative rehabilitative measure need to be investigated with future research.
Statement of ethics
This study was conducted in accordance with the Helsinki Declaration as revised in 2013. This study was reviewed and approved by University of Oklahoma Health Sciences Center Institutional Review Board. Both patients provided their written informed consent to participate in this study.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bauchet L, Mathieu-Daude H, Fabbro-Peray P, Rigau V, Fabbro M, Chinot O. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010. 12: 725-35
2. Bernhardt J, Churilov L, Ellery F, Collier J, Chamberlain J, Langhorne P. Prespecified dose-response analysis for a very early rehabilitation trial (AVERT). Neurology. 2016. 86: 2138-45
3. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T. safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016. 9: 641-61
4. Chung SW, Hill AT, Rogasch NC, Hoy KE, Fitzgerald PB. Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2016. 63: 43-64
5. Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. Measuring brain stimulation induced changes in cortical properties using TMS-EEG. Brain Stimul. 2015. 8: 1010-20
6. Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011. 7: 76-85
7. Goldsworthy MR, Pitcher JB, Ridding MC. Neuroplastic modulation of inhibitory motor cortical networks by spaced theta burst stimulation protocols. Brain Stimul. 2013. 6: 340-5
8. Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013. 67: 283-97
9. Harvey DY, Mass JA, Shah-Basak PP, Wurzman R, Faseyitan O, Sacchetti DL. Continuous theta burst stimulation over right pars triangularis facilitates naming abilities in chronic post-stroke aphasia by enhancing phonological access. Brain Lang. 2019. 192: 25-34
10. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005. 45: 201-6
11. Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke?. Lancet Neurol. 2006. 5: 708-12
12. Janicak PG, O’Reardon JP, Sampson SM, Husain MM, Lisanby SH, Rado JT. Transcranial magnetic stimulation in the treatment of major depressive disorder: A comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008. 69: 222-32
13. Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006. 117: 455-71
14. Minjoli S, Saturnino GB, Blicher JU, Stagg CJ, Siebner HR, Antunes A. The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. Neuroimage Clin. 2017. 15: 106-17
15. Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009. 10: 861-72
16. O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry. 2007. 62: 1208-16
17. Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J Clin Neurophysiol. 2011. 28: 67-74
18. Ovadia-Caro S, Khalil AA, Sehm B, Villringer A, Nikulin VV, Nazarova M. Predicting the response to non-invasive brain stimulation in stroke. Front Neurol. 2019. 10: 302
19. Pereira LS, Muller VT, da Mota Gomes M, Rotenberg A, Fregni F. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review. Epilepsy Behav. 2016. 57: 167-76
20. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009. 120: 2008-39
21. Simonyan K, Fuertinger S. Speech networks at rest and in action: Interactions between functional brain networks controlling speech production. J Neurophysiol. 2015. 113: 2967-78
22. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009. 106: 13040-5
23. Storti SF, Formaggio E, Nordio R, Manganotti P, Fiaschi A, Bertoldo A. Automatic selection of resting-state networks with functional magnetic resonance imaging. Front Neurosci. 2013. 7: 72
24. Wagner T, Eden U, Fregni F, Valero-Cabre A, RamosEstebanez C, Pronio-Stelluto V. Transcranial magnetic stimulation and brain atrophy: A computer-based human brain model study. Exp Brain Res. 2008. 186: 539-50
25. Wagner T, Fregni F, Eden U, Ramos-Estebanez C, Grodzinsky A, Zahn M. Transcranial magnetic stimulation and stroke: A computer-based human model study. Neuroimage. 2006. 30: 857-70
26. Warraich Z, Kleim JA. Neural plasticity: The biological substrate for neurorehabilitation. PM R. 2010. 2: S208-19
27. Wychowski T, Wang H, Buniak L, Henry JC, Mohile N. Considerations in prophylaxis for tumor-associated epilepsy: Prevention of status epilepticus and tolerability of newer generation AEDs. Clin Neurol Neurosurg. 2013. 115: 2365-9
28. Yu YL, Lee MS, Juan CJ, Hueng DY. Calculating the tumor volume of acoustic neuromas: Comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg. 2013. 115: 1371-4