- Department of Neurosurgery, Novosibirsk Scientific Research Institute of Traumatology and Orthopedics, Ya.L. Tsivyan, Russian Federation.
- Department of Experimental, Novosibirsk Scientific Research Institute of Traumatology and Orthopedics, Ya.L. Tsivyan, Russian Federation.
- Department of 3D Printing, LLC Logeeks MS, Novosibirsk, Russian Federation.
Correspondence Address:
Sergey Mishinov
Department of Neurosurgery, Novosibirsk Scientific Research Institute of Traumatology and Orthopedics, Ya.L. Tsivyan, Russian Federation.
DOI:10.25259/SNI_960_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sergey Mishinov1, Alexander Samokhin2, Andrey Panchenko3, Vyacheslav Stupak1. A titanium implant for Chiari malformation Type 1 surgery. 23-Feb-2021;12:72
How to cite this URL: Sergey Mishinov1, Alexander Samokhin2, Andrey Panchenko3, Vyacheslav Stupak1. A titanium implant for Chiari malformation Type 1 surgery. 23-Feb-2021;12:72. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10607
Abstract
Background: Concepts of Chiari malformation Type 1 (CM1) surgery in the present time significantly different. The most common complications are pseudomeningocele (12%) and postoperative CSF leak (5%). The development of pseudomeningocele may be associated with inappropriate restoration of bone and muscles relations.
Methods: The pilot study involved 11 patients aged 24–64 years with a diagnosis of CM1 who had indications for surgical treatment. Special titanium implant enabling fixation of the occipital and cervical muscles at the projections of their normal attachments was developed, it was placed to occipital bone on the final stages of surgical intervention. Surgical technique promoted tightened wound closure neutralizing formation of “dead space” at the place of occipital craniectomy and between muscle layers. The implant was produced by direct metal laser sintering method for each patient individually.
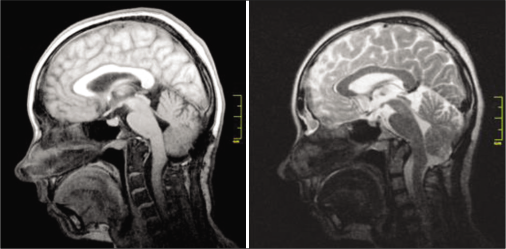
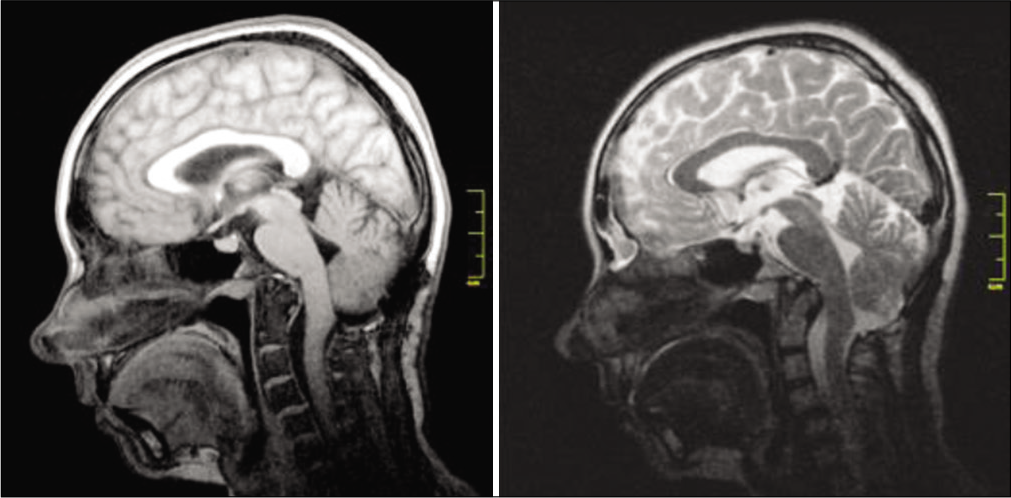
Results: There were no complications during the hospitalization and follow-up period. Postoperative MRI demonstrated adequate formation of the cisterna magna and the absence of pseudomeningocele. During follow-up period there were no signs of pseudomeningocele, CSF leak, surgical scar complications, implant-associated infections, and other complications.
Conclusion: In the study group, no pseudomeningocele cases as long as any other complications associated with surgery had been revealed. The efficacy of the proposed surgical technique using the developed implant should be evaluated in clinical trials with larger patient samples. To simplify preoperative planning and manufacturing of the implant for each patient individually, a set of implants with different specified sizes was developed.
Keywords: Acquired meningocele, Chiari malformation Type 1, Three-dimensional printing, Titanium implants
INTRODUCTION
Chiari malformation Type 1 (CM1) is herniation of the stretched cerebellar tonsils into the spinal canal through the foramen magnum, which was first described by Hans Chiari in 1891[
Aim
The aim of this pilot study was to assess rate of pseudomeningocele after CM1 surgery in adults using a specially developed titanium implant.
MATERIALS AND METHODS
Study group description
The pilot prospective, single-center, and non-randomized cohort study involved 11 patients aged 24–64 years with a diagnosis of CM1 who had indications for surgical treatment (posterior fossa correction). The study was approved by the local ethics committee of the institution. All patients provided signed informed consent. Criteria for inclusion in the study were as follows:
Age of 18–80 years, regardless of gender Indications for surgical treatment of CM1 Surgical treatment involving posterior fossa decompression, dural opening, subarachnoid revision, and formation of the cisterna magna using a dural graft Signed informed consent to participate in the pilot study.
Exclusion criteria included:
Previous surgery for CM1 Refusal to provide information about the state of health and postoperative follow-up MRI scans.
The duration of postoperative follow-up <6 months was the criterion for early withdrawal of the patient from the study.
The mean (M) age of patients was 40.7 ± 14.9 years; the distribution by gender was as follows: three (27.3%) males and 8 (72.7%) females. Before surgery, all patients underwent clinical and instrumental examination including MRI (Toshiba Vantage Titan 1.5 T.) and CT scan (Toshiba Aquilion 64) of the brain. In the presence of a syringomyelia cyst, additional MRI examination of the cervical spine was performed. Surgery was performed using a microsurgical technique with a Carl Zeiss Opmi Vario 33 microscope. On the 1st postoperative day, all patients underwent an MRI scan of the brain; if a syringomyelia cyst was present, and MRI of the cervical spine was also performed. CT scan of the brain was performed on the 7th day after surgery. In the postoperative period, all patients were followed up in the neurosurgical department. The patients were discharged from the hospital after removal of skin sutures and the formation of an adequate postoperative skin scar.
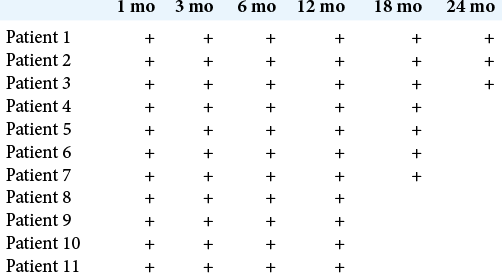
Clinical outcomes after surgery were evaluated using the Chicago Chiari Outcome Scale (CCOS). The patients were examined during follow-up visits at 1, 3, 6, 12, 18, and 24 months after surgery, with MRI of the brain being performed at 6, 12, and 24 months.
The study also included a simulation analysis group (n = 188), these patients did not participate directly in the study. The results of their tomographic examinations were used as the basis for mathematical modeling and analysis of the skull anatomical structures in relation to the designed implants.
Surgical technique
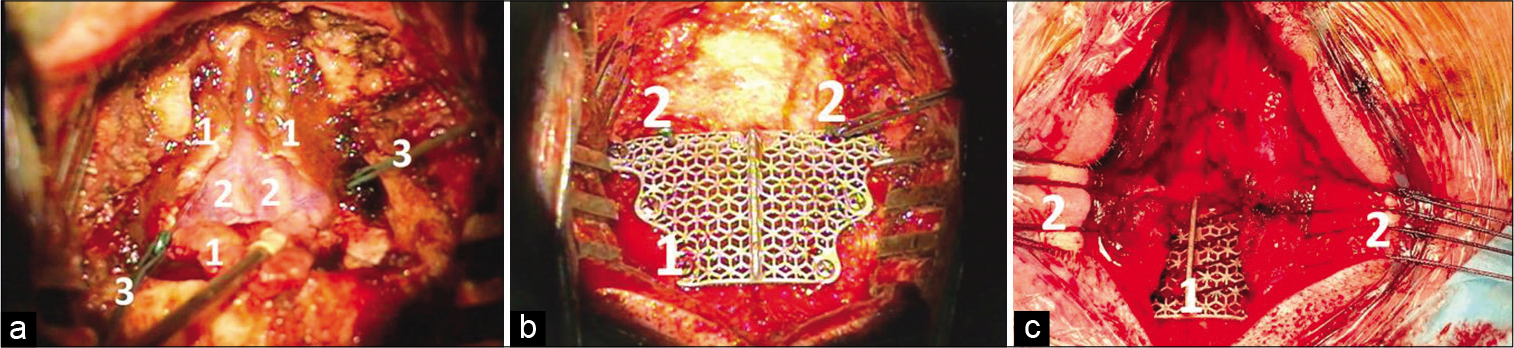
Surgery is performed under general anesthesia; the patient is placed in a concord position, with the neck flexed and the head immobilized in a Mayfield bow. At the first stage, an autologous fat graft, approximately 4–6 cm3 in volume, is harvested from the upper outer quadrant of the left gluteal region. The main stages of surgery in the posterior cranial fossa include: A midline incision in the cervical-occipital region, from the inion to C2 level; exposure of the occipital bone and C1 lamina; resection of a occipital bone portion at the square of 2 × 3 – 3 × 3 cm depending on the posterior fossa configuration and patient’s age; C1 laminectomy; dissection and partial resection of the atlanto-occipital membrane; and Y-shaped dural opening, with the base toward the transverse sinus [
Figure 1:
(a) Surgical approach to the posterior fossa. 1 – Dura mater (resected in a Y-like way), 2 – cerebellar tonsils under the arachnoid, 3 – stitches for dural tenting (b) Implant fixation and dural tenting. 1 – Titanium implant, 2 – Stitches for dural tenting (c) Closure of the posterior fossa. 1 – Titanium implant, 2 – muscle stitches.
Titanium implant
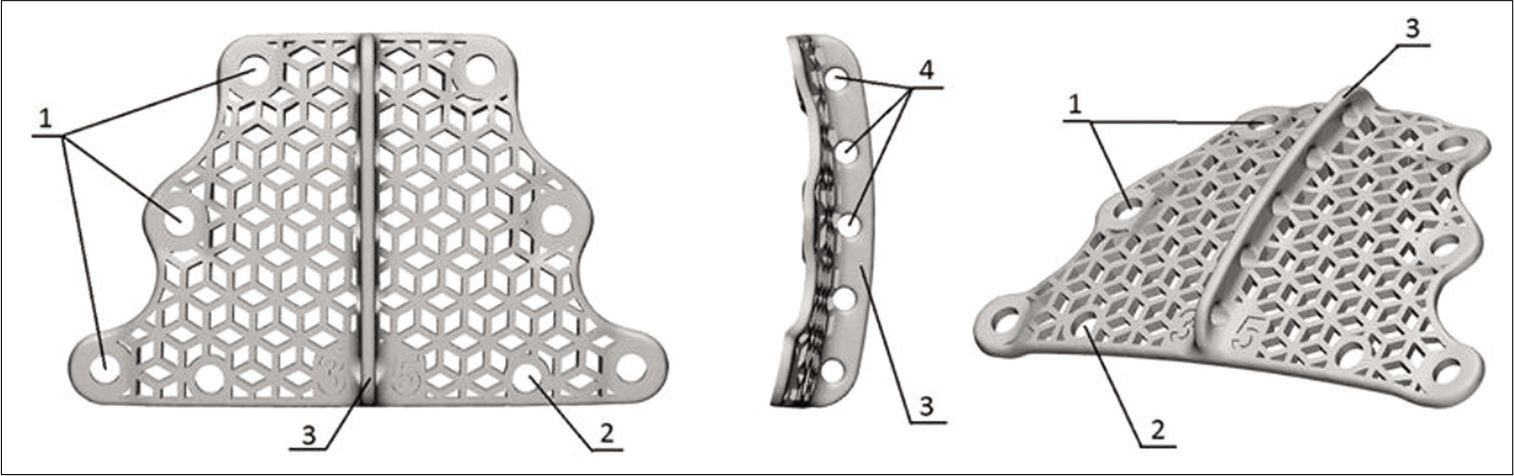
To achieve our objectives, we developed a titanium implant [
Surgical features associated with application of the implant
The surgical technique involving application of the titanium implant was modified: the dura mater is sewn with non-absorbable sutures, and ligatures of the sutures [
Assessment of implant variants
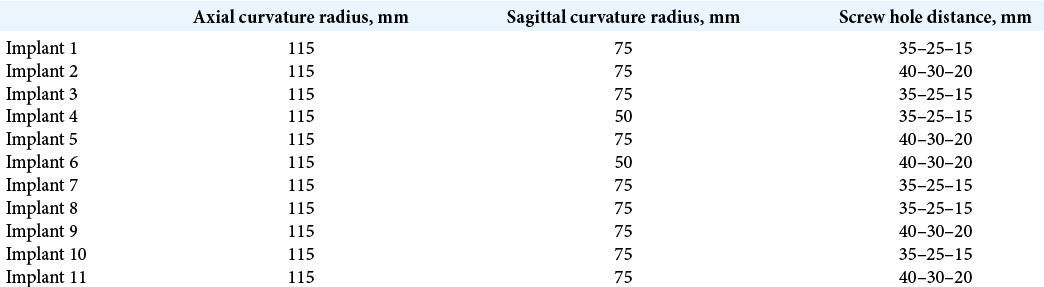
In the study, implants were prepared for each patient individually. The following implant parameters were variable: bending in the axial and sagittal plane; the distance between holes for fixation to the occipital bone. Parameters such as the mesh pattern and roughness and the vertical ridge for suturing the occipital and cervical muscles remained unchanged. Therefore, depending on the contour of the patient’s occipital bone, only the implant curvature and the distance between mounting holes were changed. The geometry of implants is presented in [
Assessment of the occipital bone structure variability (simulation analysis group)
In the study, we also assessed the variability of the occipital bone structure in the area of its resection using 3D skull models of patients who underwent previous surgery for CM1 at the neurosurgical clinic in 2005–2016 without using the titanium implant. For this, 188 preoperative CT scans of the skull of patients with this pathology were analyzed. Available images were exported in the form of a series of digital DICOM data to a program for generating a 3D model; then, the radii of the axial and sagittal curvature of the occipital bone were measured. Next, a 3D model of the skull was generated based on control postoperative radiographic scans, and the size of an occipital craniectomy defect was measured.
Statistical analysis
The data were analyzed using the Statistica 10.0 software package. In the pilot study group, descriptive statistics for scale variables were analyzed (data are presented as M and standard deviation (SD), median, minimum and maximum values, quartiles Q1 and Q3): radii of the axial and sagittal curvature, surgery duration, intraoperative blood loss, and hospital stay duration. Clinical outcomes (CCOS) and the presence of pseudomeningocele and other postoperative complications were analyzed during follow-up visits.
RESULTS
The follow-up period ranged from 30 to 9 months; the M surgery duration was 175.5 (12.5) min; intraoperative blood loss was 215.5 (197.4) ml; and hospital stay duration was 13.4 (3.5) days. There were no complications during the hospitalization period. Postoperative CT revealed tight fixation of the titanium implant to the occipital bone in all patients. Postoperative MRI demonstrated adequate formation of the cisterna magna and the absence of pseudomeningocele. After discharge from the hospital, patients underwent follow-up examinations at 1, 3, 6, 12, 18, and 24 months after surgery; MRI of the brain was performed at 6, 12, and 24 months. There were no signs of pseudomeningocele, CSF leak, surgical scar complications, implant-associated infections, and other complications. The number of postoperative visits of each patient is shown in [
An analysis of simulated implants (n = 11) revealed that variable components of the implant geometry were the sagittal curvature (radius = 50 and 70 mm) and the implant width (35–25–15 mm and 40–30–20 mm between the centers of mounting holes). Assessing the variability of the occipital bone curvature in the posterior fossa craniectomy area in patients from simulation analysis group (n = 188) found that the axial radius of the occipital curvature varied from 74 to 190 mm, with a M of 115.5 (22) mm, a median of 115 mm, Q1 of 110 mm, and Q3 of 130 mm. The sagittal radius of the occipital curvature varied from 35 to 121 mm, with a M of 63.9 (15.6) mm, a median of 61.5 mm, Q1 of 51 mm, and Q3 of 73 mm.
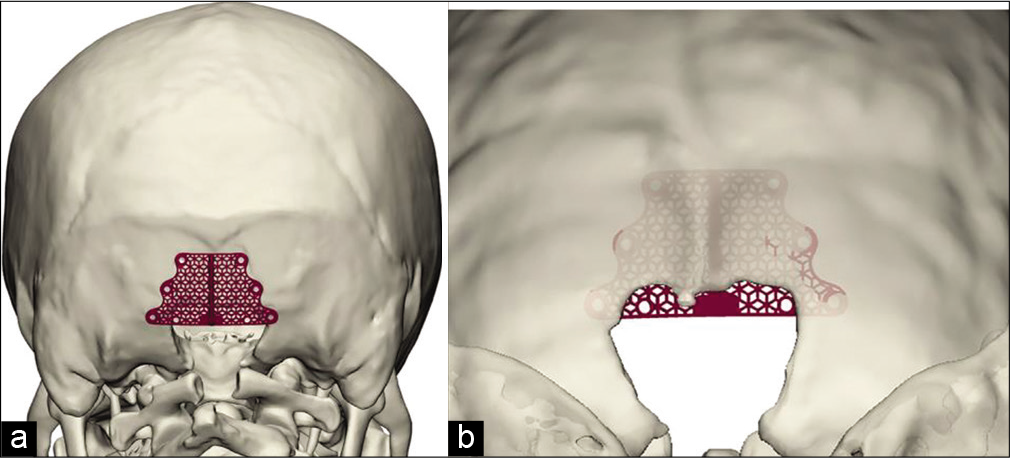
The obtained data were used to design a set of 32 implants covering all possible combinations of these parameters. Next, the stage of fixation was emulated: each of the produced titanium implant variants was matched to each virtual skull model (n = 188) in a 3D modeling environment using a simulation analysis group database. It was found that the set of typical sizes may be reduced to four variants because at least one of the implants presented in [
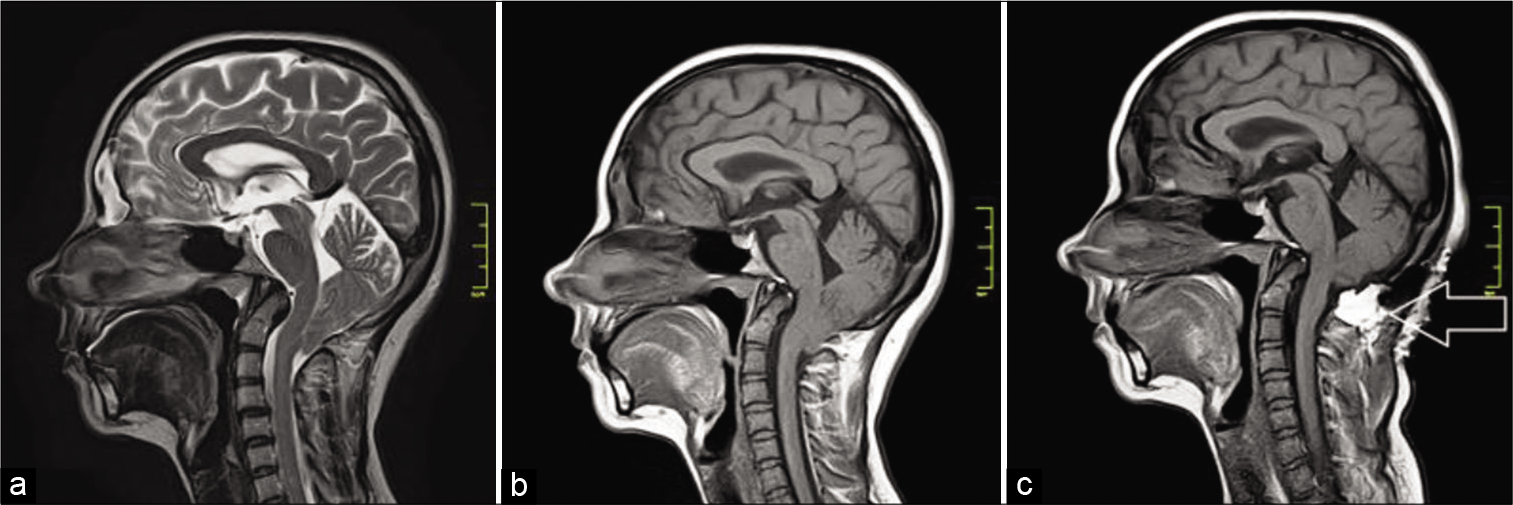
Clinical case
A 33-year-old female diagnosed with CM1 was hospitalized in April 2019 with complaints of headaches intensifying during the Valsalva test, cough headaches, and occipital and cervical pain. Preoperative MRI data are presented in
DISCUSSION
As described above, to date, there are many surgical approaches in the treatment of patients with CM1. At the final surgical stages, a number of authors have suggested using titanium mini-plates for prevention of cerebellar prolapse,[
An important aspect of any surgery is to restore the normal anatomy in the approach area as much as possible. Usually, on closing a wound at the final stages of surgery for CM1, the occipital muscles are sutured together, but not attached to the occipital bone. In this case, a small “dead space” is formed in the occipital bone resection area, which may become a cavity for accumulation of exudate or cerebrospinal fluid and subsequently progress in pseudomeningocele that accounts for about 12%,[
To prevent the development of pseudomeningocele, we has developed a titanium implant that has special elements for suturing the occipital and deep cervical muscles to their anatomical attachment sites in the area below the inion, and in addition enables dural tenting, and prevents cisterna magna compression by postoperative muscle scars. Placement of the implant was preceded by a water-tight duraplasty using synthetic grafts as well as by overlaying the dura mater with an autologous fat flap fixed by fibrin-thrombin glue.
The fat flap served as a hydrophobic seal in the formed bone defect area, which eliminated the cavity at the site of a removed occipital bone fragment. Tight suturing of the muscles to the titanium implant fixed to the occipital bone enabled restoration of the original anatomical relationships in the surgical site, namely, fixation of the muscles to the occipital bone. This manipulation also reduced the volume of intermuscular and muscular-fascial recesses. In this pilot study, no pseudomeningocele and other postoperative complications were observed.
In the pilot study group, implants were made for each patient individually, based on the anatomical structure of the occipital bone and the area of planned craniectomy. For extensive application of the proposed implants, it is advisable to develop a model set of typical sizes for intraoperative selection of the most suitable implant. The data collected out of 3D skull models from 188 patients operated previously on for CM1 were used to develop a set of implants with specified sizes [
Backward control was performed at the final stage of approval of the proposed sizes: the most appropriate implant was selected from the developed set of implants (with specified sizes and geometry) for each skull type from the simulation analysis group (n = 188) in the environment for 3D modeling.
Virtual imitation of implant fixation was performed in the occipital craniectomy area according to the described technique. On the basis of virtual modeling of surgery and selection of the most appropriate implant, it was found that at least one implant out of four from the developed set matched to each 3D model of the patient’s skull.
Identical simulation was also performed in the pilot study group, and the same results as described above had been received: at least one implant from the set matched to each 3D model of the skull. It is worth noting that 3D models of 11 patients were not taken into account upon developing the set of implants with typical sizes.
CONCLUSION
There were no pseudomeningocele cases in the pilot study group among patients operated on for CM1 using the developed titanium implant. There were also no other complications associated with surgery and the implant.
The efficacy of the proposed surgical technique using the developed implant should be evaluated in clinical trials with larger patient samples.
To simplify preoperative planning and manufacturing of the implant for each patient individually, a set of implants with different specified sizes was developed, which enabled an intraoperative choice of the optimal configuration for the patient.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alden TD, Ojemann JG, Park TS. Surgical treatment of Chiari I malformation: Indications and approaches. Neurosurg Focus. 2011. 11: E2
2. Allen KP, Isaacson B, Kutz JW, Purcell PL, Roland PS. The association of meningitis with postoperative cerebrospinal fluid fistula. J Neurol Surg B Skull Base. 2012. 73: 401-4
3. Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: A review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. 2015. 15: 161-77
4. Assina AR, Meleis AM, Cohen MA, Iqbal MO, Liu JK. Titanium mesh-assisted dural tenting for an expansile suboccipital cranioplasty in the treatment of Chiari 1 malformation. J Clin Neurosci. 2014. 21: 1641-6
5. Baisden JL. Controversies in Chiari I malformations. Surg Neurol Int. 2012. 3: S232-7
6. Batzdorf U, McArthur DL, Bentson JR. Surgical treatment of Chiari malformation with and without syringomyelia: Experience with 177 adult patients. J Neurosurg. 2013. 118: 232-42
7. Bhimani AD, Esfahani DR, Denyer S, Chiu RG, Rosenberg D, Barks AL. Adult Chiari I malformations: An analysis of surgical risk factors and complications using an international database. World Neurosurg. 2018. 115: 490-500
8. de Tommasi C, Bond AE. Complicated pseudomeningocele repair after Chiari decompression: Case report and review of the literature. World Neurosurg. 2016. 88: 688.e1-7
9. Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000. 9: e5
10. Heller JB, Lazareff J, Gabbay JS, Lam S, Kawamoto HK, Bradley JP. Posterior cranial fossa box expansion leads to resolution of symptomatic cerebellar ptosis following Chiari I malformation repair. J Craniofac Surg. 2007. 18: 274-80
11. Holly LT, Batzdorf U. Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. J Neurosurg. 2001. 1: 21-6
12. Korshunov AE, Kushel YV. Posterior decompression of the craniovertebral junction in children with Chiari malformation: A surgery extent issue. Zh Vopr Neirokhir Im N N Burdenko. 2016. 4: 11-7
13. Kushel YVKozlitina TNGlagolev NV. Method for Bone Defect Repair after Decompression Trepanation of Posterior Cranial Fossa in Patients with Anomaly of Arnold-Chiari I Type. Available from: https://www.patents.s3.yandex.net/Ru2578544c2_20160327.pdf [Last accessed on 2014 Aug 11].
14. Manley GT, Dillon W. Acute posterior fossa syndrome following lumbar drainage for treatment of suboccipital pseudomeningocele.Report of three cases. J Neurosurg. 2000. 92: 469-74
15. Mazzola CA, Fried AH. Revision surgery for Chiari malformation decompression. Neurosurg Focus. 2003. 15: E3
16. Mehendale NH, Samy RN, Roland PS. Management of pseudomeningocele following neurotologic procedures. Otolaryngol Head Neck Surg. 2004. 131: 253-62
17. Menger R, Connor DE, Hefner M, Caldito G, Nanda A. Pseudomeningocele formation following Chiari decompression: 19-year retrospective review of predisposing and prognostic factors. Surg Neurol Int. 2015. 6: 70
18. Rahman A, Rana MS, Bhandari PB, Asif DS, Uddin AN, Obaida AS. Stealth cranioplasty: A novel endeavor for symptomatic adult Chiari I patients with syringomyelia: Technical note, appraisal, and philosophical considerations. J Craniovertebr Junction Spine. 2017. 8: 243-52
19. .editors. Surgical Treatment of Chiari Malformation Type I in Adults: Clinical Recommendations. Russia, Moscow: Russian Neurosurgical Association; 2015. p.
20. Rosen DS, Wollman R, Frim DM. Recurrence of symptoms after Chiari decompression and duraplasty with nonautologous graft material. Pediatr Neurosurg. 2003. 38: 186-90
21. Steinbok P, Singhal A, Mills J, Cochrane DD, Price AV. Cerebrospinal fluid (CSF) leak and pseudomeningocele formation after posterior fossa tumor resection in children: A retrospective analysis. Childs Nerv Syst. 2007. 23: 171-4
22. Takayasu M, Takagi T, Hara M, Anzai M. A simple technique for expansive suboccipital cranioplasty following foramen magnum decompression for the treatment of syringomyelia associated with Chiari I malformation. Neurosurg Rev. 2004. 27: 173-7
23. Tu A, Tamburrini G, Steinbok P. Management of postoperative pseudomeningoceles: An international survey study. Childs Nerv Syst. 2014. 30: 1791-801
24. Udani V, Holly LT, Chow D, Batzdorf U. Posterior fossa reconstruction using titanium plate for the treatment of cerebellar ptosis after decompression for Chiari malformation. World Neurosurg. 2014. 81: 836-41
25. Williams B. A critical appraisal of posterior fossa surgery for communicating syringomyelia. Brain. 1978. 101: 223-50
26. Zide BM. How to reduce the morbidity of wound closure following extensive and complicated laminectomy and tethered cord surgery. Pediatr Neurosurg. 1992. 18: 157-66
27. Zuev AA, Pedyash NV, Epifanov DS, Kostenko GV. Results of surgical treatment of syringomyelia associated with Chiari 1 malformation.An analysis of 125 cases. Zh Vopr Neirokhir Im N N Burdenko. 2016. 80: 27-34