- College of Osteopathic Medicine, New York Institute of Technology, Glen Head, New York, United States.
- Department of Biomedical Engineering, Carle Illinois College of Medicine, University of Illinois at Urbana Champaign, Champaign, Illinois, United States.
- School of Medicine, University of New Mexico, Albuquerque, New Mexico, United States.
- Department of Neurosurgery, Desert Regional Medical Center, Palm Springs, California, United States.
Correspondence Address:
Brian Fiani
Department of Neurosurgery, Desert Regional Medical Center, Palm Springs, California, United States.
DOI:10.25259/SNI_933_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniel W. Griepp1, Jason Lee1, Christina M. Moawad2, Cyrus Davati1, Juliana Runnels3, Brian Fiani4. BIIB093 (intravenous glibenclamide) for the prevention of severe cerebral edema. 02-Mar-2021;12:80
How to cite this URL: Daniel W. Griepp1, Jason Lee1, Christina M. Moawad2, Cyrus Davati1, Juliana Runnels3, Brian Fiani4. BIIB093 (intravenous glibenclamide) for the prevention of severe cerebral edema. 02-Mar-2021;12:80. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10619
Abstract
Background: Vasogenic edema in the setting of acute ischemic stroke can be attributed to the opening of transient receptor potential 4 channels, which are expressed in the setting of injury and regulated by sulfonylurea receptor 1 (SUR1) proteins. Glibenclamide, also known as glyburide, RP-1127, Cirara, and BIIB093, is a second-generation sulfonylurea that binds SUR1 at potassium channels and may significantly reduce cerebral edema following stroke, as evidenced by recent clinical trials. This review provides a comprehensive analysis of clinical considerations of glibenclamide use and current patient outcomes when administered in the setting of acute ischemic stroke to reduce severe edema.
Methods: National databases (MEDLINE, EMBASE, Cochrane, and Google scholar databases) were searched to identify studies that reported on the clinical outcomes of glibenclamide administered immediately following acute ischemic stroke.
Results: The pharmacological mechanism of glibenclamide was reviewed in depth as well as the known indications and contraindications to receiving treatment. Eight studies were identified as having meaningful clinical outcome data, finding statistically significant differences in glibenclamide treatment groups ranging from matrix metalloproteinase-9 serum levels, midline shift, modified Rankin Scores, National Institute of Health Stroke Score, and mortality endpoints.
Conclusion: Studies analyzing the GAMES-Pilot and GAMES-PR trials suggest that glibenclamide has a moderate, however, measurable effect on intermediate biomarkers and clinical endpoints. Meaningful conclusions are limited by the small sample size of patients studied.
Keywords: BIIB093, Cerebral edema, Glibenclamide, Glyburide, Pharmacology
INTRODUCTION
Cerebral edema is a complication affecting over 70,000 patients in the United States with acute ischemic stroke each year, resulting in fatality rates approaching eighty percent.[
Cerebral edema results in the setting of increased intracranial cellular permeability due to cellular damage caused by stroke, head trauma, infection, intracranial lesions, or medication side effects.[
Glibenclamide, also known as glyburide, RP-1127, Cirara, and BIIB093, is a second-generation sulfonylurea drug that binds the SUR1 protein at potassium adenosine triphosphate channels (KATP). Although traditionally used to treat diabetes mellitus type 2, recent clinical trials have demonstrated efficacy in preventing cerebral edema in the setting of stroke. Studies have shown efficacy in inhibiting the SUR1-TRPM4 complex to prevent sodium and water influx into the cell and resulting edema.[
Patient selection
When severe or malignant edema is a complication of ischemic stroke, symptoms related to cerebral swelling specifically may be difficult to distinguish from residual deficits of stroke if eloquent regions of the brain have been affected from ischemia. Nevertheless, severe edema would be characterized by rapid decline in clinical picture, uncharacteristic of the stroke presentation. Some of the predictors and early signs of malignant edema may present as signs of increased intracranial pressure such as depressed consciousness, gaze palsy, and vomiting.[
In the clinical pilot studies first investigating the use of glibenclamide for cerebral edema, hypoglycemia was a significant concern given the primary use of this pharmacotherapy for diabetes. However, despite close monitoring of blood glucose throughout the course of study, hypoglycemia was not reported to contribute to poorer outcomes in the setting of acute ischemic stroke.[
At present, there are no clear contraindications for use in pregnancy. Glibenclamide has been shown to cross the placenta in pregnancy and exposure to the drug in utero has been reported, however, the drug is digested by placental microsomes and toxicity in utero has not been reported.[
Recent study of development of glibenclamide for the prevention of cerebral edema following acute ischemic stroke has been primarily through intravenous (IV) administration.[
Given that glibenclamide for the application of ischemic stroke is indicated on an emergent basis, it is important to screen for obvious contraindications before initiating treatment. However, as with any therapy, the benefits of life-saving treatment must be weighed against contraindications that may result in undesirable sequalae but are more concerning with long-term use.
Pharmacology
SUR1-TRPM4 channels, previously referred to as SUR1-regulated NCCa-ATP channels, have been shown to play a critical role in the formation of cytotoxic edema. Normally, such channels regulate against a pathological rise in intracellular calcium during brain injury; SUR1-TRPM4 channels also are sensitive to the intracellular concentration of ATP.[
In ischemic or traumatic brain cells, binding of glibenclamide to SUR1-TRPM4 reduces depolarization which reduced blood-brain barrier (BBB) leakage and the formation of cerebral edema.[
Physiologically, glibenclamide and other sulfonylureas do not accumulate in the brain, even though there are neurons that express KATP channels in the CNS.[
MATERIALS AND METHODS
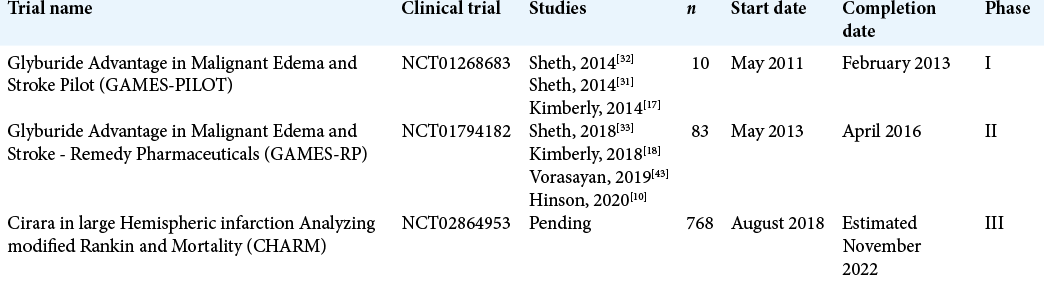
To identify investigational use of glibenclamide, a literature search of national databases was performed to identify studies that reported objective outcomes when used in the setting of stroke. Studies were also identified that could provide insight into the clinical use and specific indications of glibenclamide. Eight studies published over the past 6 years were identified as having meaningful data that looked at clinical outcomes of glibenclamide use in acute ischemic stroke [
Trials and outcomes
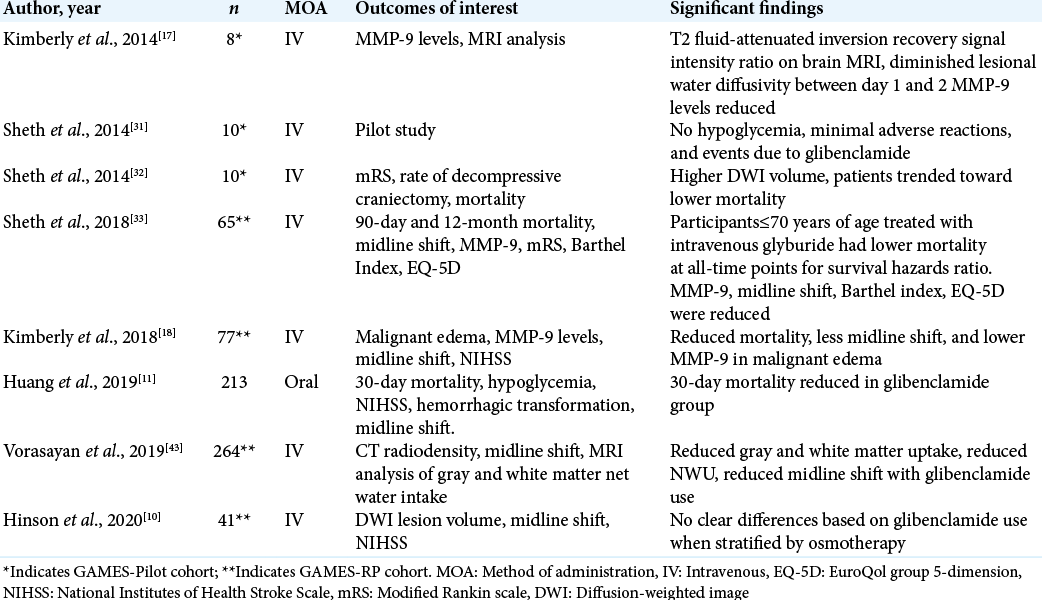
Previous clinical studies have demonstrated that IV glibenclamide has resulted in reduction in plasma MMP-9 levels, which are typically elevated following stroke and indicate several stroke-related complications, particularly brain edema after stroke.[
In further analyzing the changes in the brain with IV glibenclamide in the GAMES-RP cohort, Vorasayan et al. conducted a post-hoc exploratory analysis using a modified intention-to-treat sample.[
Another important direct result of cerebral edema is reflected in the quantitative measure of midline shift, which describes displacement of cerebral structures laterally and has been correlated with diminished and often irreversible poor mental status, especially when measures such as decompressive craniectomy are not pursued.[
Another study was identified investigating the use of glibenclamide similarly in acute ischemic stroke, however, administered orally, not IV.[
In the discussion of long-term outcomes and quality of life differences that may be apparent due to treatment with glibenclamide, four studies investigated the functional outcomes of patients who suffered ischemic stroke and were subsequently treated with glibenclamide compared with placebo controls. Outcomes of interest included a modified Rankin Scale (mRS), Barthel index, EuroQol group 5-dimension (EQ-5D), NIHSS, and finally mortality. Sheth et al. described the outcomes of patients included in the GAMES-Pilot cohort of ten patients in terms of 90-day mRS compared with matched controls, finding that patient’s treatment with glibenclamide has a higher proportion of 0–4 mRS (P = 0.049, Fisher’s exact test).[
With regard to mortality, glibenclamide was not shown to reduce mortality in the GAMES-Pilot study; however, statistically significant difference in mortality was achieved in analysis of the GAMES-RP cohort.[
CONCLUSION
This review provides, to date, the largest and most comprehensive review of the use of glibenclamide for use in acute ischemic stroke with analysis of measurable outcomes of MMP-9, midline shift, mRS, NIHSS, and mortality. Literature not included in this analysis was minimal and reported on the investigational use of glibenclamide in the setting of traumatic brain injury, which was not reviewed in depth.[
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003. 34: 325-37
2. da Costa BB, Windlin IC, Koterba E, Yamaki VN, Rabelo NN, Solla DJ. Glibenclamide in aneurysmatic subarachnoid hemorrhage (GASH): Study protocol for a randomized controlled trial. Trials. 2019. 20: 413
3. Ding J, Yuan F, Guo JY, Chen H, Tian HL. Influence of glibenclamide on outcome in patients with Type 2 diabetes and traumatic brain injury. Clin Neurol Neurosurg. 2013. 115: 2166-9
4. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011. 8: 344-56
5. Foerch C, Montaner J, Furie KL, Ning MM, Lo EH. Invited article: Searching for oracles? Blood biomarkers in acute stroke. Neurology. 2009. 73: 393-9
6. Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: A comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007. 30: 389-94
7. Gerzanich V, Kwon MS, Woo SK, Ivanov A, Simard JM. SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One. 2018. 13: e0195526
8. .editors. Glibenclamide: A review. Drugs. 1971. 1: 116-40
9. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008. 359: 1317-29
10. Hinson HE, Sun E, Molyneaux BJ, von Kummer R, Demchuk A, Romero J. Osmotherapy for malignant cerebral edema in a phase 2 prospective, double blind, randomized, placebo-controlled study of IV glibenclamide. J Stroke Cerebrovasc Dis. 2020. 29: 104916
11. Huang K, Hu Y, Wu Y, Ji Z, Wang S, Lin Z. Exploratory analysis of oral glibenclamide in acute ischemic stroke. Acta Neurol Scand. 2019. 140: 212-8
12. Jha RM, Desai SM, Zusman BE, Koleck TA, Puccio AM, Okonkwo DO. Downstream TRPM4 polymorphisms are associated with intracranial hypertension and statistically interact with ABCC8 polymorphisms in a prospective cohort of severe traumatic brain injury. J Neurotrauma. 2019. 36: 1804-17
13. Jha RM, Puccio AM, Chou SH, Chang CH, Wallisch JS, Molyneaux BJ. Sulfonylurea receptor-1: A novel biomarker for cerebral edema in severe traumatic brain injury. Crit Care Med. 2017. 45: e255-64
14. Jha SK. Cerebral edema and its management. Med J Armed Forces India. 2003. 59: 326-31
15. Kawoos U, McCarron RM, Auker CR, Chavko M. Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. Int J Mol Sci. 2015. 16: 28979-97
16. Khalili H, Derakhshan N, Niakan A, Ghaffarpasand F, Salehi M, Eshraghian H. Effects of oral glibenclamide on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injuries: A randomized double-blind placebo-controlled clinical trial. World Neurosurg. 2017. 101: 130-6
17. Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014. 20: 193-201
18. Kimberly WT, Bevers MB, von Kummer R, Demchuk AM, Romero JM, Elm JJ. Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial. Neurology. 2018. 91: e2163-9
19. Kimber-Trojnar Z, Marciniak B, Patro-Malysza J, SkorzynskaDziduszko K, Poniedzialek-Czajkowska E, Mierzynski R. Is glyburide safe in pregnancy?. Curr Pharm Biotechnol. 2014. 15: 100-12
20. Liang D, Bhatta S, Gerzanich V, Simard JM. Cytotoxic edema: Mechanisms of pathological cell swelling. Neurosurg Focus. 2007. 22: E2
21. Loh KP, Ng G, Yu CY, Fhu CK, Yu D, Vennekens R. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. 2014. 466: 563-76
22. Mannucci E, Monami M, Candido R, Pintaudi B, Targher G. SID-AMD Joint Panel for Italian Guidelines on Treatment of Type 2 Diabetes, Effect of insulin secretagogues on major cardiovascular events and all-cause mortality: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020. 30: 1601-8
23. Mehta AB. Glucose-6-phosphate dehydrogenase deficiency. Postgrad Med J. 1994. 70: 871-7
24. Mehta RI, Ivanova S, Tosun C, Castellani RJ, Gerzanich V, Simard JM. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2013. 72: 871-83
25. Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol. 2012. 235: 282-96
26. Pallan TV, Ahmed I. Glyburide in treating malignant cerebral edema, blocking sulfonyl urea one (SUR1) receptors. J Vasc Interv Neurol. 2014. 7: 23-5
27. Pergakis M, Badjatia N, Chaturvedi S, Cronin CA, Kimberly WT, Sheth KN. BIIB093 (IV glibenclamide): An investigational compound for the prevention and treatment of severe cerebral edema. Expert Opin Investig Drugs. 2019. 28: 1031-40
28. Raee MR, Nargesi AA, Heidari B, Mansournia MA, Larry M, Rabizadeh S. All-cause and cardiovascular mortality following treatment with metformin or glyburide in patients with Type 2 diabetes mellitus. Arch Iran Med. 2017. 20: 141-6
29. Serena J, Blanco M, Castellanos M, Silva Y, Vivancos J, Moro MA. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005. 36: 1921-6
30. Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016. 15: 1160-9
31. Sheth KN, Kimberly WT, Elm JJ, Kent TA, Mandava P, Yoo AJ. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014. 45: 281-3
32. Sheth KN, Kimberly WT, Elm JJ, Kent TA, Yoo AJ, Thomalla G. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit Care. 2014. 21: 43-51
33. Sheth KN, Petersen NH, Cheung K, Elm JJ, Hinson HE, Molyneaux BJ. Long-term outcomes in patients aged ≤70 years with intravenous glyburide from the Phase II GAMES-RP study of large hemispheric infarction: An exploratory analysis. Stroke. 2018. 49: 1457-63
34. Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006. 12: 433-40
35. Simard JM, Sheth KN, Kimberly WT, Stern BJ, del Zoppo GJ, Jacobson S. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2014. 20: 319-33
36. Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010. 41: 531-7
37. Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch. 2012. 464: 573-82
38. Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015. 11: 840-8
39. Sorby-Adams AJ, Marcoionni AM, Dempsey ER, Woenig JA, Turner RJ. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int J Mol Sci. 2017. 18: 1788
40. Szeto V, Chen NH, Sun HS, Feng ZP. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin. 2018. 39: 683-94
41. Tinker A, Aziz Q, Li Y, Specterman M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol. 2018. 8: 1463-511
42. Tomiyama Y, Brian JE, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: Role of ATP-sensitive potassium channels. Stroke. 1999. 30: 1942-7
43. 43. Vorasayan P, Bevers MB, Beslow LA, Sze G, Molyneaux BJ, Hinson HE. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke. 2019. 50: 3021-7
44. Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013. 288: 3655-67
45. Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X. Early prediction of malignant brain edema after ischemic stroke. Stroke. 2018. 49: 2918-27
46. Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011. 42: 3323-8
47. Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W. A protective role of glibenclamide in inflammation-associated injury. Mediators Inflamm. 2017. 2017: 3578702