- Department of Neurosurgery, Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
- Department of Imaging Diagnostics Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
- Department of Angiology, Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
DOI:10.25259/SNI_170_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toma Yuriev Spiriev1, Milko Milev1, Lili Laleva1, Stoicho Stoyanov1, Ivan Plachkov2, Milena Staneva3, Vladimir Nakov1. A rare case of carotid body tumor associated with near complete cerebral sinus thrombosis and idiopathic intracranial hypertension. Management strategy and review of the literature. 07-Jun-2021;12:262
How to cite this URL: Toma Yuriev Spiriev1, Milko Milev1, Lili Laleva1, Stoicho Stoyanov1, Ivan Plachkov2, Milena Staneva3, Vladimir Nakov1. A rare case of carotid body tumor associated with near complete cerebral sinus thrombosis and idiopathic intracranial hypertension. Management strategy and review of the literature. 07-Jun-2021;12:262. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10865
Abstract
Background: Carotid body tumors (CBTs) are rare hypervascular lesions with critical location which makes them very challenging to treat. In rare occasions, compression of the jugular vein from the tumor mass could predispose to progressive thrombosis of intracranial venous sinuses. The latter consequently leads to intracranial hypertension (pseudotumor cerebri) with the accompanying danger to the vision. Herewith, we present our management strategy for this rare presentation of CBTs.
Case Description: A 38-year-old woman, with no medical history, was admitted in the emergency unit with acute onset of headache, dizziness, and vomiting. On the diagnostic imaging studies (CT venography and MRI) a near total occlusion of all cerebral venous sinuses and a large CBT (Shambin Type II) were diagnosed. Initially, the patient was treated with anticoagulants for the thrombosis and with lumbo-peritoneal (LP) shunt for the management of pseudotumor cerebri. At a second stage, after resolution of the cerebral sinus thrombosis, the CBT was completely resected under electrophysiological monitoring, without preoperative embolization. At 1-year follow-up, the patient is neurologically intact with functioning LP shunt, patent cerebral venous sinuses, without tumor recurrence.
Conclusion: We present a rare case of CBT with intracranial complications, which was managed successfully by staged treatment. Careful study of the preoperative radiological and laboratory data, thorough preoperative planning of the tridimensional lesion anatomy, as well as meticulous microsurgical technique under intraoperative electrophysiological monitoring was essential for the successful outcome of the case.
Keywords: Carotid body tumor, Cerebral dural venous thrombosis, Idiopathic intracranial hypertension, Intraoperative electrophysiological monitoring, Pseudo tumor cerebri, Tridimensional planning, Horos software
INTRODUCTION
Carotid body tumors (CBTs) or carotid paragangliomas are complex vascular lesions, which are derived from chromaphin cells within the adventitia of the carotid bifurcation. These tumors account for 0.6% of all head and neck tumors and with incidence of 1:30,000–1:100,000 in the general population.[
CASE DESCRIPTION
A 38-years-old woman was admitted in the emergency department with severe onset of headache, nausea, and vomiting. On the performed computed tomography (CT) and magnetic resonance imaging (MRI) angio- and venography, there was thrombosis of both transverse and the left sigmoid sinuses, thrombosis of sinus rectus and superior sagittal sinus, and associated brain edema [
Figure 1:
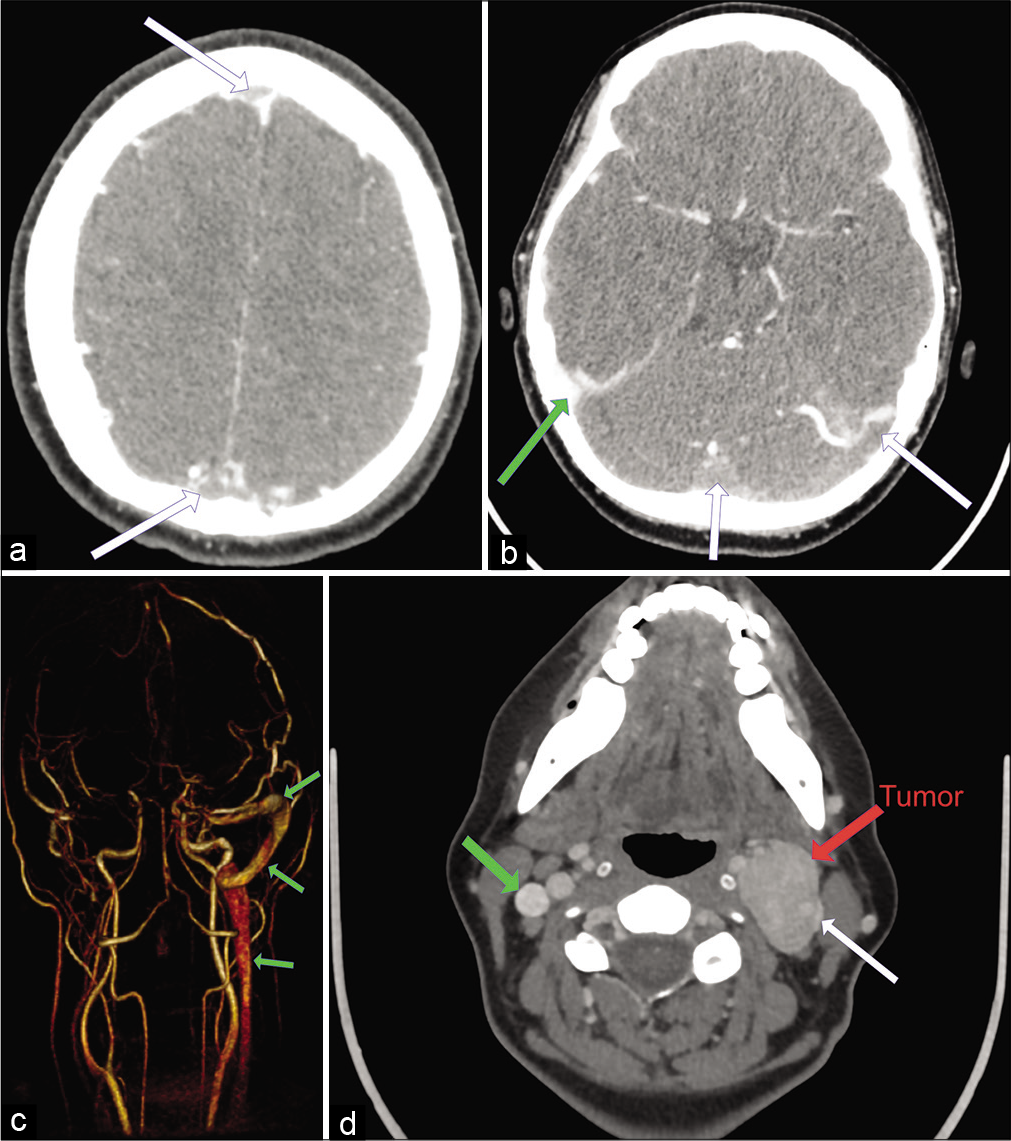
(a) Preoperative Computed Tomography (CT) venography indicating thrombosis of the superior sagittal sinus (white arrow) (b) preoperative CT venography indicating thrombosis of the confluens sinuum, left sigmoid and transverse sinuses (white arrow). The right sigmoid sinus is patent (green arrow). (c) Preoperative magnetic resonance imaging angiography (posterior view) indicating the extent of the intracranial thrombosis. Only the right transverse and sigmoid sinuses are patent (green arrow). (d) CT venography indicating the tumor location (left carotid bifurcation, white arrow) and the associated internal jugular vein compression. The right internal jugular vein is patent (green arrow).
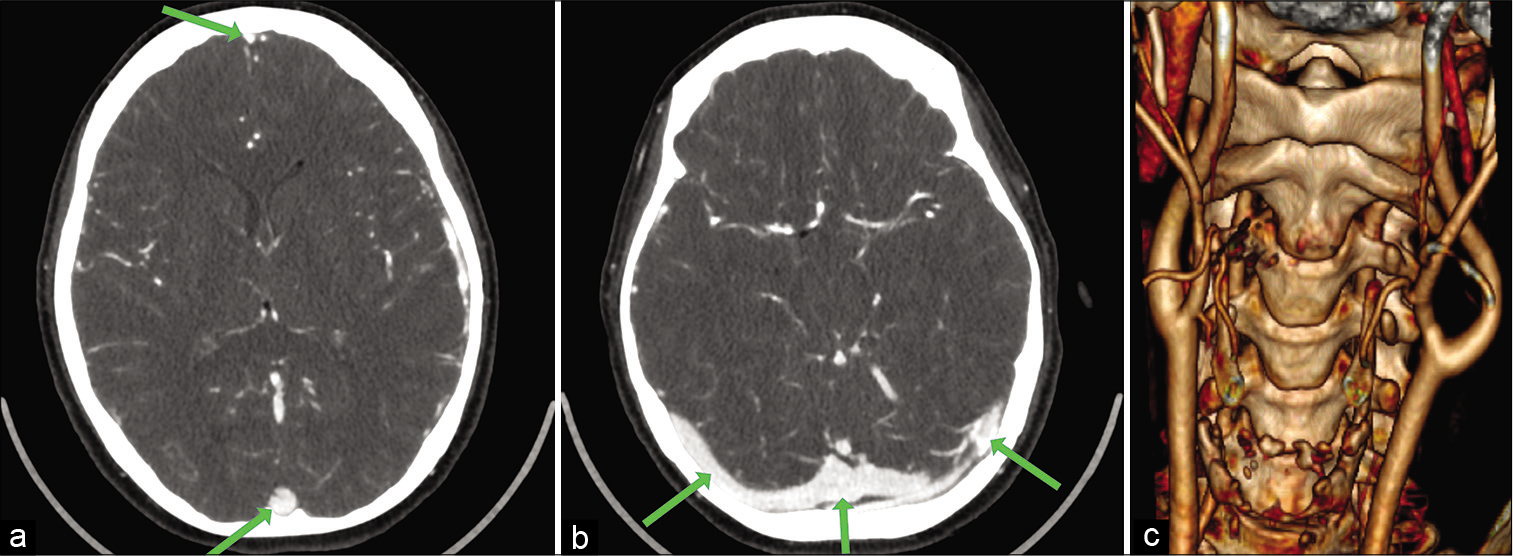
A control MRI was done 1 month after the first hospitalization indicating complete resolution of the cerebral venous thrombosis. The patient was scheduled for surgery of the CBT almost 2 months after the initial onset of symptoms. The surgery was planned on CT and MRI angiography [
Figure 2:
(a) Preoperative computed tomography based three-dimensional reconstruction with Horos™ software (an open source 64-bit medical image viewer available at:
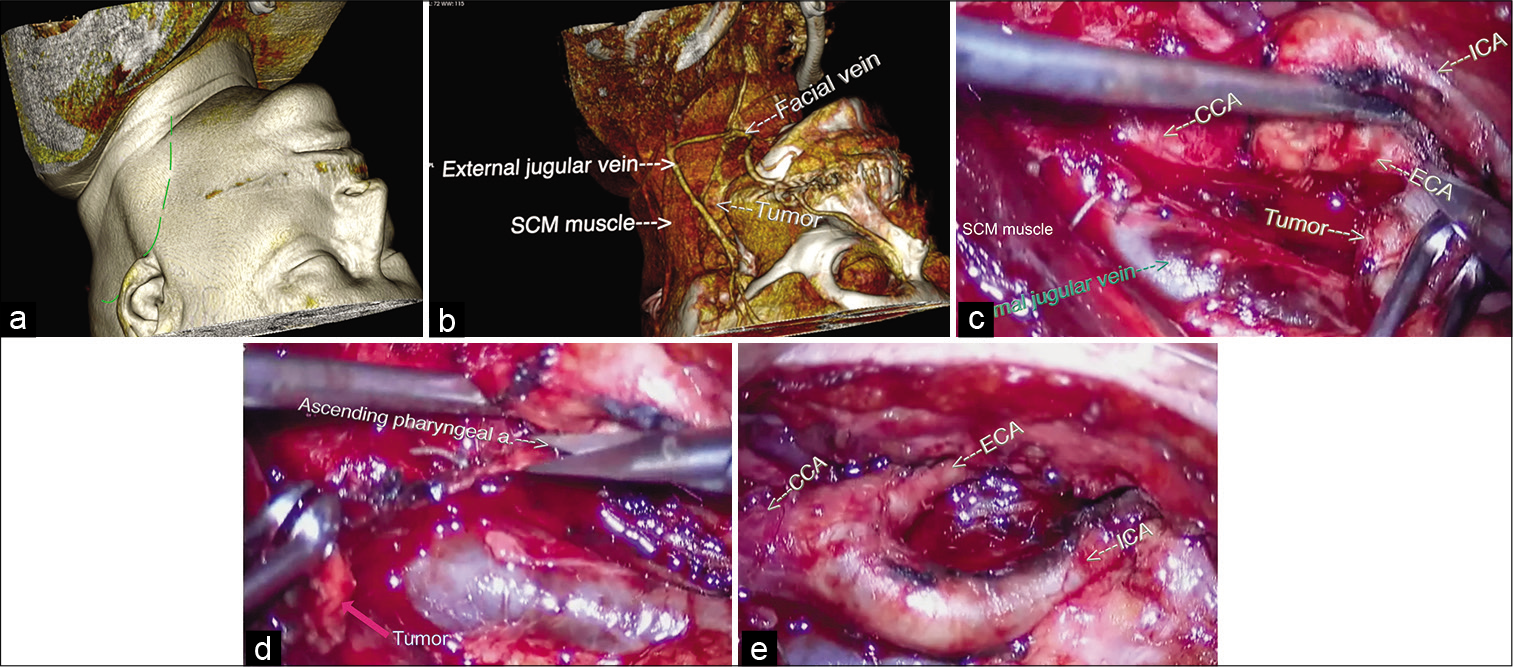
The surgery was performed under intraoperative electrophysiological monitoring which included somatosensory evoked potentials (SSEP) and cortico-spinal and cortico-bulbar motor evoked potentials (MEP), direct nerve stimulation for the identification of the IX, X, XI, and XII nerves and bispectral index monitoring. The surgical protocol was as follows: the patient was in prone position on the operative table with head rotated and extended to the right side, fixed on the Mayfield head clamp. A reverse “J” incision was made starting from the middle third of the medial border of the sternocleidomastoid muscle (SCM) [
Video 1
Figure 4:
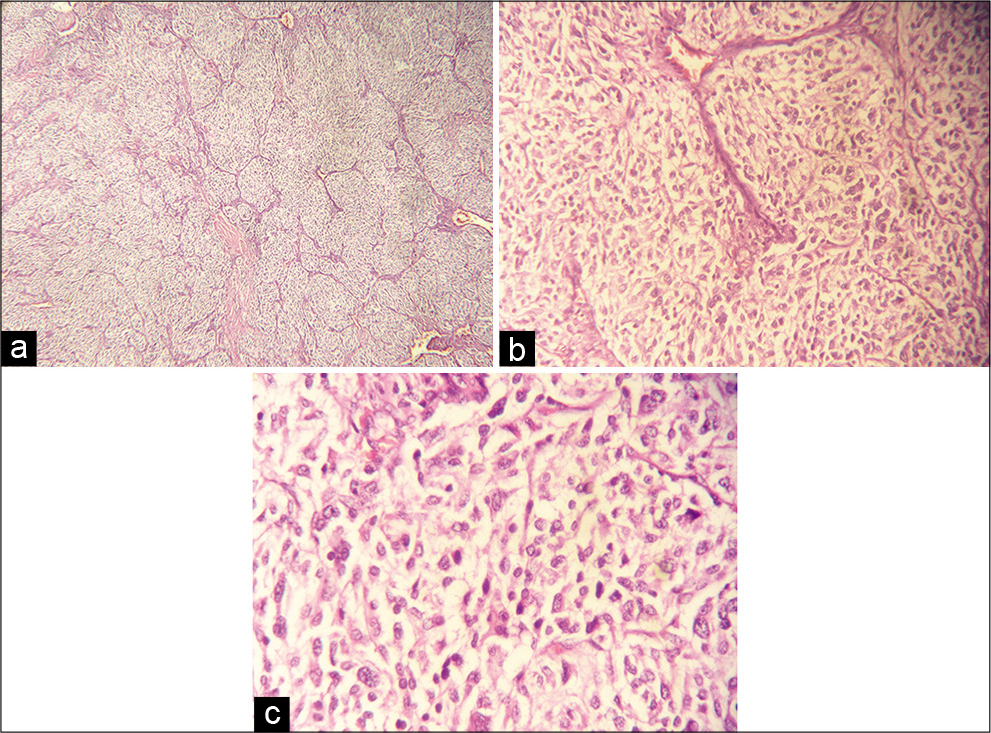
(a-c) Micrograph of the pathological specimen with hematoxylin and eosin stain presenting typical arrangement of the tumor cells in cell balls (Zellballen), separated by fibrotic stroma and vessels. The cells are oval or polygonal with abundant granular eosinophilic cytoplasm and nuclear atypia.
The genetic tests for thrombophilia indicated that the patient is homozygotic for Factor V Leiden and PAI-1 4G/5G wild type allele. However, she was found to be a heterozygotic carrier of the G20210A prothrombin gene mutation, and compound heterozygosity for the C677T and A1298C MTHFR mutations was further identified. Therefore, due to the high risk of future thrombotic events, the oral anticoagulant (rivaroxaban in a daily dosage of 20 mg) treatment was continued, and the patient was followed up with MRI and neck vessels Doppler ultrasound examinations.
After a 1-year follow-up the patient is neurologically intact with functioning LP shunt, without new thrombosis of the cerebral sinuses, without tumor recurrence.
DISCUSSION
Idiopathic intracranial hypertension or pseudotumor cerebri is a serious condition characterized by decreased absorption of cerebrospinal fluid due to cerebral venous outflow obstruction, which, if not managed accordingly, could lead to progressive visual loss [
The treatment strategies include cerebral spinal fluid (CSF) diversion procedures (LP or ventriculoperitoneal [VP] shunting), optic sheath fenestration in cases with progressive visual deterioration or, recently, cerebral venous sinuses stenting (in chronic cases of sinus stenosis).[
CSF shunting is one of the main options for treatment of the increased intracranial pressure from venous outflow obstruction and idiopathic intracranial hypertension. [
There is no consensus in the literature for preference of certain type of shunting in the case of pseudotumor cerebri.[
Despite our best efforts, we have found few papers in the literature related to such cases of extensive cerebral venous sinus thrombosis associated with CBT. This does not mean that this is the first case, but, most probably, that the condition is underreported. The main papers that we found are case reports and small case series for jugular paraganglioma patients presenting with increased intracranial pressure due to venous outflow obstruction by the tumor.[
CBTs are very challenging to treat due to their hypervascularity, location within the carotid bifurcation and proximity to major cranial nerves (IX, X, and XII nerves). In such cases, as a part of the preoperative planning and avoidance of vascular complications, the patient was monitored for eloquent brain functions with salvage strategy for intra-extracranial bypass. Operation was performed under advanced electrophysiological monitoring, which includes SSEP and corticospinal and cortico-bulbar MEP, which would detect any form of cerebral ischemia as well as ongoing injury to the caudal group of cranial nerves. Direct nerve stimulation was also employed allowing for the identification of the IX, X, XI, and XII cranial nerves, aiding with their preservation. One of the main risks in this type of surgery is cranial nerves morbidity with estimated rate between 14% and 49% incidence of early neurological deficit and 6–23% – for persistent deficit at 1 year follow-up according to the literature.[
Preoperative CT and MRI angiography provided the most important information regarding the diagnosis and the choice of treatment paradigm. The protocol included CT venography, which indicated the extent of internal jugular vein and cerebral venous sinuses thrombosis. From a preoperative planning perspective, this data were used for three-dimensional (3D) reconstruction and planning with Horos™ software (an open source 64-bit medical image viewer available at:
Preoperative angiography was not performed just as preoperative tumor embolization was not considered after the initial imaging examinations, as we believe that this would not have added any benefit to the surgical plan in relation to blood loss or better preoperative understanding of pathological anatomy. This is also supported by other authors[
CONCLUSION
Idiopathic intracranial hypertension is a rare presentation of a CBT. In the case described, it was due to advanced thrombosis of the cerebral venous sinuses resulting from stretching and compression of the jugular vein along with genetic predisposition for thrombophilia. Prompt treatment of the venous thrombosis and intracranial hypertension followed by second stage microsurgical excision of the tumor mass without preoperative embolization under intraoperative electrophysiological monitoring prevented further neurological complications and proved to be a successful management strategy in this case.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on:
www.surgicalneurologyint.com
References
1. Abu-Ghanem S, Yehuda M, Carmel NN, Abergel A, Fliss DM. Impact of preoperative embolization on the outcomes of carotid body tumor surgery: A meta-analysis and review of the literature. Head Neck. 2016. 38: E2386-94
2. Abubaker K, Ali Z, Raza K, Bolger C, Rawluk D, O’Brien D. Idiopathic intracranial hypertension: Lumboperitoneal shunts versus ventriculoperitoneal shunts-case series and literature review. Br J Neurosurg. 2011. 25: 94-9
3. Almawi WY, Tamim H, Kreidy R, Timson G, Rahal E, Nabulsi M. A case control study on the contribution of factor V-Leiden, prothrombin G20210A, and MTHFR C677T mutations to the genetic susceptibility of deep venous thrombosis. J Thromb Thrombolysis. 2005. 19: 189-96
4. Amato B, Serra R, Fappiano F, Rossi R, Danzi M, Milone M. Surgical complications of carotid body tumors surgery: A review. Int Angiol. 2015. 34: 15-22
5. Bai C, Ding J, Da Z, Sun J, Liu C, Pan L. Probable risk factors of internal jugular vein stenosis in Chinese patients-a real-world cohort study. Clin Neurol Neurosurg. 2020. 191: 105678
6. Beck DW, Kassell NF, Drake CG. Glomus jugulare tumor presenting with increased intracranial pressure. Case report. J Neurosurg. 1979. 50: 823-5
7. Can Sevil F, Tort M, Ali Kaygin M. Carotid body tumor resection: Long term outcome of 67 cases without preoperative embolization. Ann Vasc Surg. 2020. 67: 200-7
8. Chung I, Lip GY. Virchow’s triad revisited: Blood constituents. Pathophysiol Haemost Thromb. 2003. 33: 449-54
9. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010. 41: 2575-80
10. Davila VJ, Chang JM, Stone WM, Fowl RJ, Bower TC, Hinni ML. Current surgical management of carotid body tumors. J Vasc Surg. 2016. 64: 1703-10
11. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F. European stroke organization guideline for the diagnosis and treatment of cerebral venous thrombosis-endorsed by the European academy of neurology. Eur J Neurol. 2017. 24: 1203-13
12. Friedman DI. The pseudotumor cerebri syndrome. Neurol Clin. 2014. 32: 363-96
13. Gao M, Feng N, Zhang M, Ti X, Zuo X. Meta-analysis of the relationship between methylenetetrahydrofolate reductase C677T and A1298C polymorphism and venous thromboembolism in the Caucasian and Asian. Biosci Rep. 2020. 40: BSR20200860
14. Giridharan N, Patel SK, Ojugbeli A, Nouri A, Shirani P, Grossman AW. Understanding the complex pathophysiology of idiopathic intracranial hypertension and the evolving role of venous sinus stenting: A comprehensive review of the literature. Neurosurg Focus. 2018. 45: E10
15. Lamblin E, Atallah I, Reyt E, Schmerber S, Magne JL, Righini CA. Neurovascular complications following carotid body paraganglioma resection. Eur Ann Otorhinolaryngol Head Neck Dis. 2016. 133: 319-24
16. Lertakyamanee P, Srinivasan A, de Lott LB, Trobe JD. Papilledema and vision loss caused by jugular paragangliomas. J Neuroophthalmol. 2015. 35: 364-70
17. Liu F, Silva D, Malone MV, Seetharaman K. MTHFR A1298C and C677T polymorphisms are associated with increased risk of venous thromboembolism: A retrospective chart review study. Acta Haematol. 2017. 138: 208-15
18. Lowe GD. Virchow’s triad revisited: Abnormal flow. Pathophysiol Haemost Thromb. 2003. 33: 455-7
19. Markey K, Mollan S, Jensen R, Sinclair A. Understanding idiopathic intracranial hypertension: Mechanisms, management, and future directions. Lancet Neurol. 2016. 15: 78-91
20. McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: Predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004. 101: 627-32
21. Orru E, Gursoy M, Gailloud P, Blitz AM, Carey JP, Olivi A. Jugular vein invasion rate in surgically operated paragangliomas: A multimodality retrospective study. Clin Imaging. 2014. 38: 815-20
22. Power AH, Bower TC, Kasperbauer J, Link MJ, Oderich G, Cloft H. Impact of preoperative embolization on outcomes of carotid body tumor resections. J Vasc Surg. 2012. 56: 979-89
23. Purvin VA, Trobe JD, Kosmorsky G. Neuro-ophthalmic features of cerebral venous obstruction. Arch Neurol. 1995. 52: 880-5
24. Robertson V, Poli F, Hobson B, Saratzis A, Naylor AR. A systematic review and meta-analysis of the presentation and surgical management of patients with carotid body tumours. Eur J Vasc Endovasc Surg. 2019. 57: 477-86
25. Sajid MS, Hamilton G, Baker DM. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007. 34: 127-30
26. Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2015. 36: 1899-904
27. Semenov S, Abalmasov V. Semiotics of lesions of the cerebral venous collectors on application of noninvasive techniques of X-ray diagnosis. Vestn Rentgenol Radiol. 2001. 5: 9-15
28. Simone B, De Stefano V, Leoncini E, Zacho J, Martinelli I, Emmerich J. Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210A and methylenetethraydrofolate reductase C677T: A meta-analysis involving over 11, 000 cases and 21, 000 controls. Eur J Epidemiol. 2013. 28: 621-47
29. Spiriev T, Nakov V, Laleva L, Tzekov C. OsiriX software as a preoperative planning tool in cranial neurosurgery: A step-by-step guide for neurosurgical residents. Surg Neurol Int. 2017. 8: 241
30. Wilson M, Browne JD, Martin T, Geer C. Case report: Atypical presentation of jugular foramen mass. Am J Otolaryngol. 2012. 33: 370-4