- Department of Neurosurgery, University of Iowa,
- Carver College of Medicine, University of Iowa, Iowa City, Iowa, United States.
Correspondence Address:
Colin Gold, Department of Neurosurgery, University of Iowa, Iowa City, Iowa, United States.
DOI:10.25259/SNI_276_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Colin Gold1, Ioannis Kournoutas2, Scott C. Seaman1, Jeremy Greenlee1. Bone flap management strategies for postcraniotomy surgical site infection. 12-Jul-2021;12:341
How to cite this URL: Colin Gold1, Ioannis Kournoutas2, Scott C. Seaman1, Jeremy Greenlee1. Bone flap management strategies for postcraniotomy surgical site infection. 12-Jul-2021;12:341. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10967
Abstract
Background: Surgical site infection (SSI) after a craniotomy is traditionally treated with wound debridement and disposal of the bone flap, followed by intravenous antibiotics. The goal of this study is to evaluate the safety of replacing the bone flap or performing immediate titanium cranioplasty.
Methods: All craniotomies at single center between 2008 and 2020 were examined to identify 35 patients with postoperative SSI. Patients were grouped by bone flap management: craniectomy (22 patients), bone flap replacement (seven patients), and titanium cranioplasty (six patients). Retrospective chart review was performed to identify patient age, gender, index surgery indication and duration, diffusion restriction on MRI, presence of gross purulence, bacteria cultured, sinus involvement, implants used during surgery, and antibiotic prophylaxis/ treatment. These variables were compared to future infection recurrence and wound breakdown.
Results: There was no significant difference in infection recurrence or future wound breakdown among the three bone flap management groups (P = 0.21, P = 0.25). None of the variables investigated had any significant relation to infection recurrence when all patients were included in the analysis. However, when only the bone flap replacement group was analyzed, there was significantly higher infection recurrence when there was frank purulence present (P = 0.048).
Conclusion: Replacing the bone flap or performing an immediate titanium cranioplasty is safe alternatives to discarding the bone flap after postoperative craniotomy SSI. When there is gross purulence present, caution should be used in replacing the bone flap, as infection recurrence is significantly higher in this subgroup of patients.
Keywords: Bone flap, Cranioplasty, Craniotomy, Infection, Postoperative complication, Surgical site infection
INTRODUCTION
Infection after a craniotomy is an uncommon but highly morbid complication that creates a predicament: what to do with the bone flap? Fortunately, postcraniotomy infection rates are low, with ranges between 1% and 11%.[
Management of a surgical site infection (SSI) typically involves operative debridement, wound culture, and subsequent antibiotics.[
Historically, craniectomy has been the favored technique for debridement of an infection. However, there is significant morbidity associated with explanting the bone flap. Aside from the inherent risks of a skull defect, there is significant risk associated with a staged cranioplasty, including: infection: 3.1–26.4%,[
Conventionally, cranioplasty is delayed until refractory intracranial hypertension has resolved, the skin is well healed and vascularized, and systemic/cranial infections are treated; typically 3 months after a hemicraniectomy.[
However, another study found that patients who had a cranioplasty within 14 days of the initial craniectomy had increased risk of infection and that 15–30 days were ideal due to lower infection, seizure, and bone resorption rates. The only benefit identified in waiting more than 90 days was a reduced rate of hydrocephalus.[
Finally, studies have shown that up to 50% of bone flaps cultured at time of elective, “sterile” craniotomy before replacement in the same surgery had positive cultures.[
Our aim is to retrospectively evaluate our institutional outcomes with infected craniotomy bone flaps, spanning the aforementioned management strategies.
MATERIALS AND METHODS
Institutional Review Board approval was obtained to identify patients who had undergone a craniotomy and subsequently, a cranial wound debridement surgery with the Neurosurgery Department between 2008 and 2020. Included patients were those who underwent an initial craniotomy for any reason and then had a subsequent debridement surgery. To be included, the wound cultures from the debridement had to be positive or infectious disease had to determine that an infection warranting treatment was present despite negative wound cultures. Retrospective chart review was performed to identify multiple patient and surgical characteristics. Patient information was recorded including age, gender, weight, and diabetes mellitus diagnosis. The all-encompassing list of craniotomies between July 2008 and July 2020 included 1730 surgeries. Thirty-five patients were included in the final analysis, after the total craniotomy list was cross-matched with the list of cranial wound debridement surgeries and patients without a true infection were eliminated. The nature of the index surgery was also investigated. These variables included indication for initial surgery, index surgery operative time, sinus involvement, largest craniotomy diameter, number of days of antibiotic prophylaxis, and use of implants. Subsequent patient treatment variables included radiation therapy and chemotherapy. Variables pertaining to the ensuing infection included the clinical presentation after infection, imaging features, gross appearance intraoperatively, bone flap management strategy, pathogen identified, and antibiotic management. The depth of infection was determined by both radiographic evidence and gross appearance during surgery. The four groups of infection depths were subgaleal, epidural, subdural, and intraparenchymal. “Frank purulence” was documented if empyema was observed in any of the aforementioned compartments. All bone flaps re-implanted at the time of debridement surgery were thoroughly irrigated with antibiotic-containing irrigation. All patients had a subgaleal drain placed at the time of surgery that was kept in place postoperatively until the drain output was zero. The outcomes that were investigated were infection recurrence and future wound breakdown, and future re-operation.
Intraoperative management strategies were of three types, so patients were grouped accordingly. Group 1: discarding of the bone flap without reconstruction (i.e. craniectomy) and delayed cranioplasty, Group 2: discarding of the bone flap with immediate titanium cranioplasty, and Group 3: washing the bone flap and immediate re-implantation. Re-implanted bone flaps were cleaned using a pulse irrigator with 3L of vancomycin in saline and then soaked in vancomycin-saline solution until re-implantation.
Statistical analysis
All statistical analysis was performed using SPSS (SPSS Inc., IBM, Chicago, IL). Chi-square tests were used to compare infection recurrence with bone flap management, future wound breakdown with bone flap management strategy, diabetes status, and index surgery indication with infection recurrence. When the minimum expected counts were <5, Fisher’s exact test was used for a 2 × 2 table and Likelihood ratio was used for any table larger than 2 × 2. One-way ANOVA was used compare length of hospital stay among bone flap management groups. Independent samples t-tests were used to compare index surgery operative time, therapeutic antibiotic duration, craniotomy diameter, prophylactic antibiotic duration, and age with infection recurrence. We divided Gram stain results into Gram positive, Gram negative, mixed, and no growth and performed a binary logistic regression to correlate Gram stain results with infection recurrence.
RESULTS
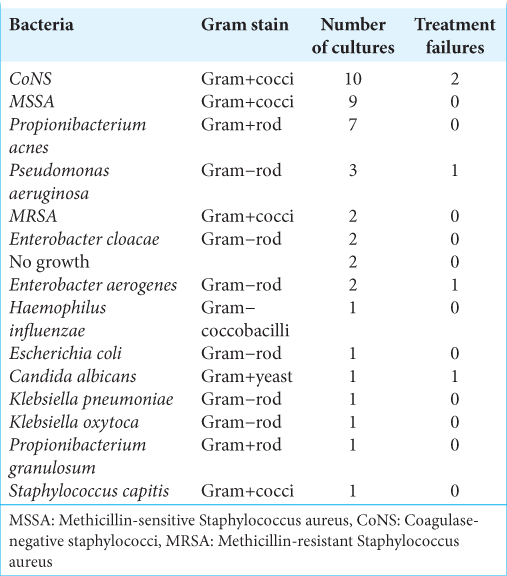
Our institution’s craniotomy infection rate has been 2.0% over the study period. The mean age of the included patients was 48 years with the youngest being 5 and the oldest being 76. There were 22 males (63%) and 13 females (37%). The mean follow-up time was 25.5 months with the minimum being 2 months and the maximum being 111 months. The indications for the index surgery were distributed as follows: meningioma 31%, glioma 20%, metastasis 23%, trauma 6%, cranioplasty 6%, hemorrhage 3%, inflammatory 3%, and other 9%. Twenty-two patients (63%) had a craniectomy during the debridement surgery, seven patients (20%) had the bone flap washed and replaced, and six patients (17%) had titanium implanted. There were four patients who had recurrent infection after initial debridement. There were 14 different pathogens identified from debridement cultures, with some patients growing multiple species of pathogen. The most common species identified were coagulase-negative Staphylococcus, which was identified in ten cultures, methicillin-sensitive Staphylococcus aureus, which was identified in nine cultures, and Propionibacterium acnes, found in seven cultures [
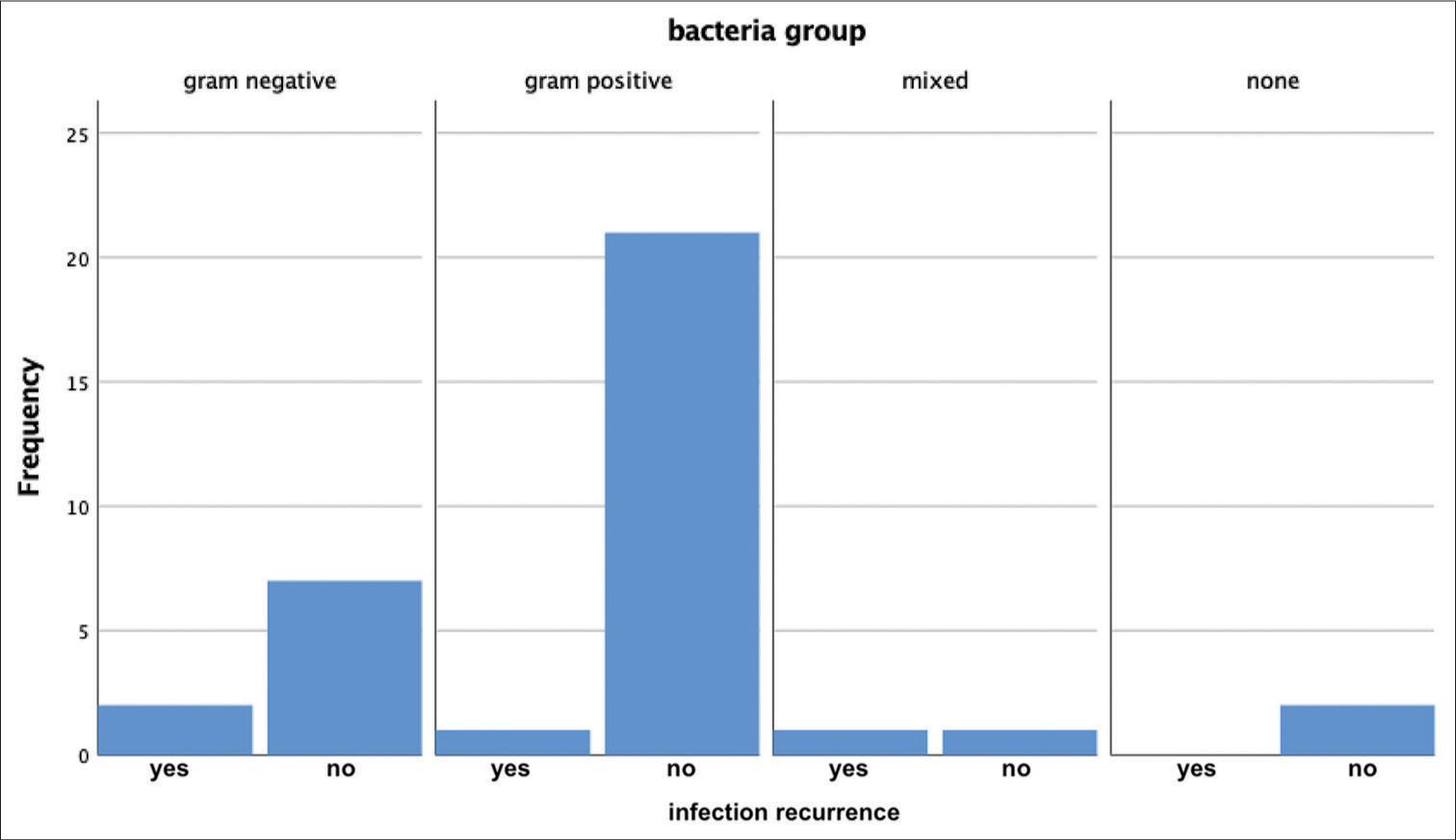
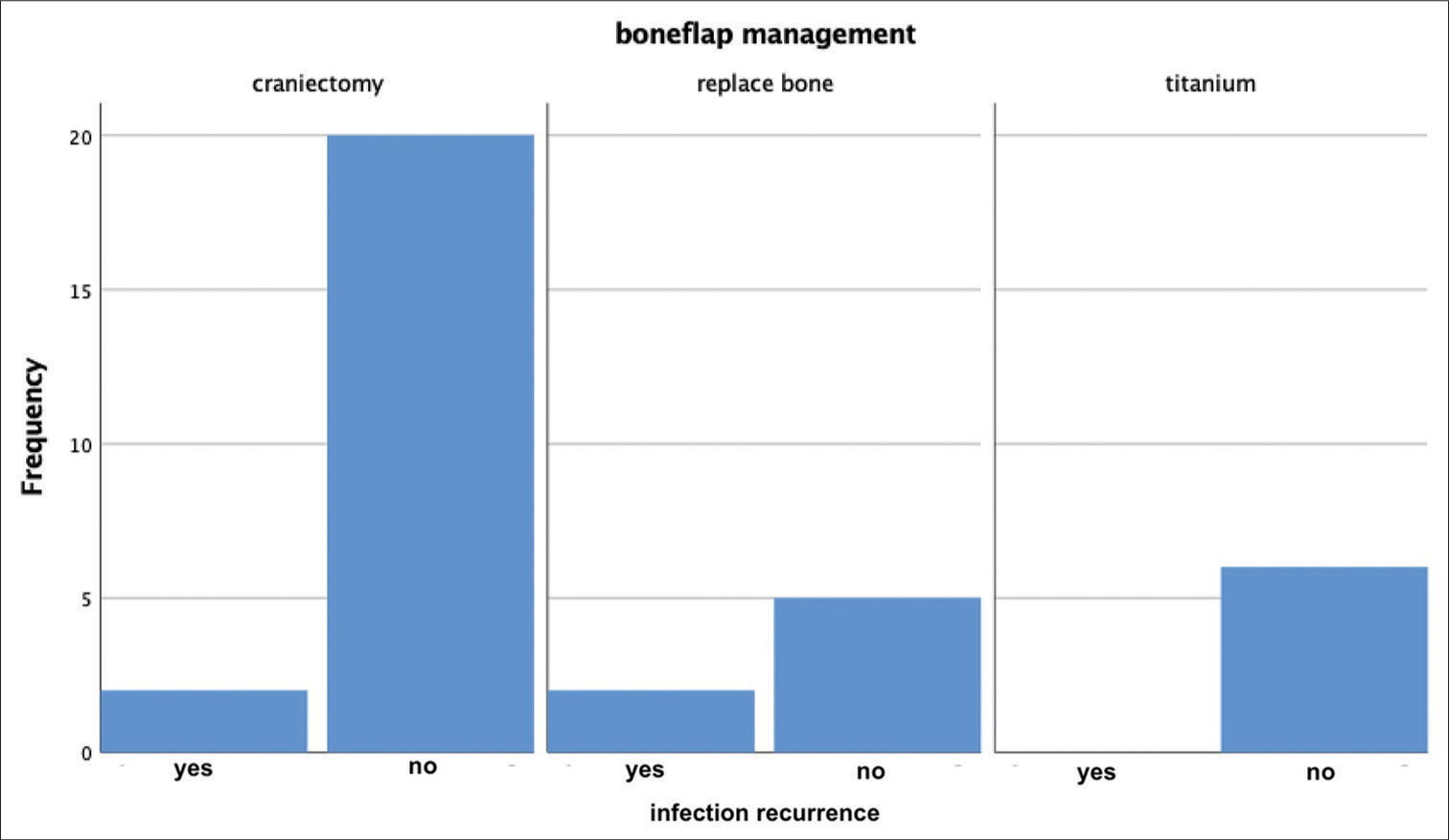
Four total patients had persistent or recurrent infection after initial treatment. Two of these patients had a craniectomy at the time of the initial debridement and two had the bone flap washed and replaced [
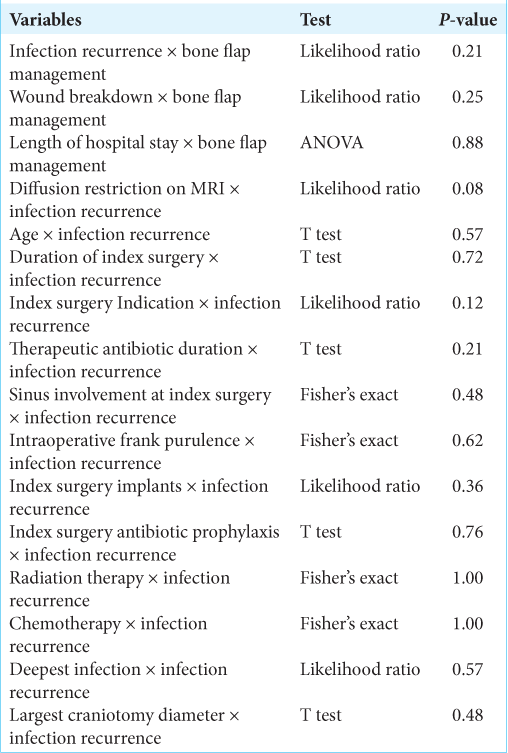
We also considered intra-operative gross appearance in relation to infection recurrence. When dividing the patients who had a debridement surgery by the presence of frank purulence, we found no difference in infection recurrence (P = 0.62) when all bone flap management strategies were included in the study. However, when we only included patients who had the bone flap washed and replaced, there was a significant association between the presence of frank purulence and infection recurrence (P = 0.048). Of the seven patients who had the bone flap washed and replaced, both who had frank purulence developed infection recurrence, while none of the five patients who did not show frank purulence had infection recurrence. A summary of the variables considered, statistical test and P-values are shown in
DISCUSSION
The results of our study were similar to the results of prior investigators in that there was no significant difference between the traditional standard of care after a postcraniotomy infection, craniectomy, and other bone flap management strategies such as washing and replacing the bone and immediate titanium cranioplasty. There was no difference in rates of reinfection or future wound breakdown amongst the three groups in our analysis. Interestingly, the only significant relationship to recurrent infection identified was in subgroup analysis of patients who had the bone flap washed and replaced. Patients who had gross purulence noted during the debridement surgery and then had the bone flap washed and replaced had significantly higher infection recurrence rates than those who did not have evidence of purulent material. This same association was not noted in the group who underwent craniectomy at the time of the debridement. No patients in the group who had titanium implanted at the time of the debridement had evidence of gross purulence. In addition, none of the patients who underwent immediate titanium cranioplasty developed recurrent infection.
The established paradigm for the management of postcraniotomy SSI is to debride the surgical bed, obtain cultures, discard the infected bone flap, treat with targeted antibiotics, and eventually perform a cranioplasty.[
Many of these other studies did not comment on the presence of gross purulence at the time of debridement; however, Wallace et al., 2018, reported that all patients in an 11-patient series where the bone flap was washed and replaced had “frankly contaminated” bone flaps. One out of 11 of these patients had an indolent infection that was incidentally noted at the time of re-operation for rapid tumor recurrence.[
Only six patients in the current study had an immediate titanium cranioplasty and none of those patients had gross purulence present during the debridement. Kshettry et al., 2012, showed in a prospective study of 24 patients who all underwent immediate titanium cranioplasty that there were zero persistent infections and higher patient satisfaction.[
Each of the studies mentioned above had treatment failures and cited the risk factors that they felt led to recurrent infection. The more commonly cited risk factors include prior radiation and chemotherapy, index surgery involving the nasal sinuses or mastoids, prior cranioplasty, and CSF leak.[
Prior research at our institution demonstrated that 50% of patients undergoing a craniotomy or craniectomy had positive bacterial cultures from swabs taken of the bone flap intraoperatively. Despite the positive cultures, there was no significant difference found in infection rate after reimplanting those bone flaps either during the craniotomy or during staged cranioplasty.[
The limitations of this study primarily pertain to the number of patients included. Although 35 total patients are large for a study of a rare entity such as postoperative craniotomy SSI, the number of patients in the bone flap replacement group (seven patients) and titanium cranioplasty group (six patients) is small. The only variable found to significantly associate with infection recurrence was presence of frank purulence in the bone flap replacement subgroup. While it may be difficult to draw conclusions from subgroup analysis with only seven patients, all of the patients who had gross purulence in this group developed recurrent infection, while none of the patients without frank purulence had infection recurrence. One potential method to work around small patient numbers for a rare disease entity is to do a multi-center study in the future.
Besides conducting larger studies, another future avenue of inquiry is the investigation of the utility of adjuvant therapies alongside the management strategies discussed above. One example is hyperbaric O2, a treatment modality has been shown to stimulate microbial killing by phagocytes, as well as enhancing wound-healing. While hyperbaric O2 is not currently in widespread use, it may work synergistically with one of the above SSI treatment options and improve patient outcomes beyond what is currently feasible.[
CONCLUSION
Postoperative SSI after a craniotomy is a dreaded complication that has traditionally been managed by operative debridement with bone flap removal. This treatment strategy involves performing a delayed cranioplasty, an operation that carries significant morbidity. Our study demonstrates that there is no difference in infection recurrence rate when the bone flap is discarded, washed, and replaced, or an immediate titanium cranioplasty is performed. However, clinicians should use caution in choosing to replace the bone flap when there is frank purulence present. Our study showed a significant increase in infection recurrence when the bone flap was replaced in the face of gross purulence.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akhaddar A.editors. Cranial Osteomyelitis: Diagnosis and Treatment. Cham: Springer; 2016. p.
2. Auguste KI, McDermott MW. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigation system. Technical note and case series of 12 patients. J Neurosurg. 2006. 105: 640-4
3. Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008. 122: 195e-208e
4. Bruce JN, Bruce SS. Preservation of bone flaps in patients with post-craniotomy infections. J Neurosurg. 2003. 98: 1203-7
5. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
6. Chiang HY, Steelman VM, Pottinger JM, Schlueter AJ, Diekema DJ, Greenlee JD. Clinical significance of positive cranial bone flap cultures and associated risk of surgical site infection after craniotomies or craniectomies. J Neurosurg. 2011. 114: 1746-54
7. Chibbaro S, di Rocco F, Mirone G, Fricia M, Makiese O, di Emidio P. Decompressive craniectomy and early cranioplasty for the management of severe head injury: A prospective multicenter study on 147 patients. World Neurosurg. 2011. 75: 558-62
8. Chou SN, Erickson DL. Craniotomy infections. Clin Neurosurg. 1976. 23: 357-62
9. de Bonis P, Frassanito P, Mangiola A, Nucci CG, Anile C, Pompucci A. Cranial repair: How complicated is filling a. “hole”?. J Neurotrauma. 2012. 29: 1071-6
10. Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Galacho-Harriero AM, Rodríguez-Salazar A. Preservation of bone flap after craniotomy infection. Neurocirugia (Astur). 2009. 20: 124-31
11. Farrell CJ, Pisculli ML, Barker FG, Winn HR.editors. Postoperative Infections of the Head and Brain. Youmans Neurological Surgery. Philadelphia, PA: WB Saunders; 2011. p. 560-9
12. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9
13. Honeybul S, Ho KM. Cranioplasty: Morbidity and failure. Br J Neurosurg. 2016. 30: 523-8
14. Kshettry VR, Hardy S, Weil RJ, Angelov L, Barnett GH. Immediate titanium cranioplasty after debridement and craniectomy for postcraniotomy surgical site infection. Neurosurg. 2012. 70: 8-14
15. Morton RP, Abecassis IJ, Hanson JF, Barber JK, Chen M, Kelly CM. Timing of cranioplasty: A 10.75-year single-center analysis of 754 patients. J Neurosurg. 2017. 128: 1648-52
16. O’Keeffe AB, Lawrence T, Bojanic S. Oxford craniotomy infections database: A cost analysis of craniotomy infection. Br J Neurosurg. 2012. 26: 265-9
17. Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012. 10: 268-72
18. Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG. Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011. 2: 123
19. Stephens FL, Mossop CM, Bell RS, Tigno T, Rosner MK, Kumar A. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. 2010. 28: E3
20. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62
21. Wallace DJ, McGinity MJ, Floyd JR. Bone flap salvage in acute surgical site infection after craniotomy for tumor resection. Neurosurg Rev. 2018. 41: 1071-7
22. Whitmore RG, Danish SF, Grady MS, Winn HR.editors. Cranioplasty. Youmans Neurological Surgery. Philadelphia, PA: WB Saunders; 2011. p. 481-6
23. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M. Complications following cranioplasty: Incidence and predictors in 348 cases. J Neurosurg. 2015. 123: 182-8