- Department of Neurosurgery, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Neuropsychiatric Research, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Neurophysiological Research, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Pediatric Neurosurgery, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Neuroophthalmological Research, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Neuropathology, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of X-ray and Radioisotope Research, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
- Department of Neuroanesthesiology, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
Correspondence Address:
Baiyr Dombaanai, Department of Neurosurgery, Burdenko National Medical Research Center of Neurosurgery, Moscow, Russia.
DOI:10.25259/SNI_350_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: David Pitskhelauri1, Elina Kudieva1, Maria Kamenetskaya2, Antonina Kozlova3, Pavel Vlasov4, Baiyr Dombaanai1, Natalia Eliseeva5, Lyudmila Shishkina6, Alexander Sanikidze1, Evgeniy Shults7, Dmitriy Moshev8, Igor Pronin7, Armen Melikyan4. Multiple hippocampal transections for mesial temporal lobe epilepsy. 27-Jul-2021;12:372
How to cite this URL: David Pitskhelauri1, Elina Kudieva1, Maria Kamenetskaya2, Antonina Kozlova3, Pavel Vlasov4, Baiyr Dombaanai1, Natalia Eliseeva5, Lyudmila Shishkina6, Alexander Sanikidze1, Evgeniy Shults7, Dmitriy Moshev8, Igor Pronin7, Armen Melikyan4. Multiple hippocampal transections for mesial temporal lobe epilepsy. 27-Jul-2021;12:372. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11001
Abstract
Background: The purpose of this study was to evaluate the effectiveness of multiple hippocampal transections (MHT) in the treatment of drug-resistant mesial temporal lobe epilepsy.
Methods: Six patients underwent MHT at Burdenko Neurosurgery Center in 2018. The age of the patients varied from 18 to 43 years. All patients suffered from refractory epilepsy caused by focal lesions of the mesial temporal complex or temporal pole in dominant side. Postoperative pathology revealed neuronal-glial tumors in two patients, focal cortical dysplasia (FCD) of the temporal pole – in two patients, cavernous angioma – in one patient, and encephalocele of the preuncal area – in one patient.
Results: All patients underwent surgery satisfactorily. There were no postoperative complications except for homonymous superior quadrantanopia. This kind of visual field loss was noted in four cases out of six. During the follow-up period five patients out of six had Engel Class I outcome (83.3%). In one case, seizures developed after 1 month in a patient with FCD in the uncus (Engel IVA). After surgery, three out of six patients developed significant nominative aphasia. Two patients relative to the preoperative level demonstrated improvement in delayed verbal memory after MHT. Two patients showed a decrease level in delayed verbal memory. In preoperative period, visual memory was below the normal in one patient. Delayed visual memory in two cases impaired compared to the preoperative level.
Conclusion: MHT can be considered as an effective method of drug-resistant mesial temporal lobe epilepsy caused by tumors of the medial temporal complex. At the same time, MHT makes it possible to preserve memory in patients with structurally preserved hippocampus. However, MHT do not guarantee the preservation of memory after surgery.
Keywords: Epilepsy surgery, Hippocampal transections, Memory, Multiple hippocampal transection, Neuronalglial tumor
INTRODUCTION
Resective surgery is an effective treatment for drug-resistant mesial temporal lobe epilepsy.[
However, the consequences of resection of the hippocampus, where its function is still preserved, can be a decrease in verbal memory or visual-spatial memory, intelligence, emotional and speech performance, as well as cognitive disorders.[
To solve this problem, Shimizu et al.[
It is established that the hippocampus has two types of pathways: (1) trisynaptic pathways, which are located in parallel loops oriented orthogonally to the longitudinal axis of the hippocampus; and (2) longitudinal pathways that run along the long axis of the hippocampus.[
This paper analyzes the results of six surgeries of patients with drug-resistant medial temporal lobe epilepsy.
MATERIALS AND METHODS
Patients population
From March 2018 to June 2018, six patients underwent MHT at Burdenko Neurosurgery Center [
Patient’s selection
In this group, we included the patients who suffered from drug-resistant epilepsy caused by focal lesions of the mesial temporal complex. Although, no clear signs of changes in the architecture and volume of the hippocampus were found on MRI scans. In all cases, the lesion was localized in the dominant left hemisphere. In all patients, memory was intact or slightly reduced. Final decision to perform the hippocampal transection was made intraoperatively – if epileptiform activity was recorded on the hippocampus following the resection of the lesion of the temporal lobe, transection was performed.
The decision to perform a surgical treatment was made at an interdisciplinary committee of physicians with the participation of neurosurgeons, neurologists, neurophysiologists, neuropsychologists, and neuroradiologists.
Patient’s examination
In all cases, video EEG showed seizures initiating from the left temporal lobe. Stereo EEG was not performed in any case. MRI revealed the lesions in the left dominant hemisphere but significant structural abnormalities did not extend to the hippocampus. Intraoperative electrocorticography (ECoG) of the basal temporal lobe and hippocampus was performed. ECoG recordings were performed during the background recording and after each stage of surgery (resection of the lesion, resection of the uncus with amygdala, and transection of the hippocampus). The recordings were made on an electroencephalograph “NicoletOne” (V44, USA).
The median follow-up after surgery comprised 27 months (from 25 to 31 months). Seizure outcome was evaluated using Engel scale.[
Neuropsychological tests before surgery and 6 months after surgery were obtained in all patients. Neuropsychological tests included the assessment of immediate and delayed verbal memory (evaluated using the Rey Auditory Verbal Learning Test - RAVLT), immediate and delayed visuospatial memory (evaluated using the Brief Visuospatial Memory Test–Revised - BVMT–R), motor memory, assessment of the preservation of executive functions (evaluated using the Frontal Assessment Battery - FAB) and spatial representations (evaluated using the Judgment of Line Orientation - JLO). In addition, a comprehensive, qualitative neuropsychological examination of higher mental functions was carried out to exclude specific disorders of gnosis, praxis, and speech. Before surgery, five out of six patients had normal values. In one patient (F29), there was a moderate decrease in scores on all the assessment scales.
All patients underwent an examination of the visual fields by means of automatic static perimetry, both before and after the surgery on the Humphrey II - 730 visual field analyzer.
The study was approved by the local ethical committee of Burdenko Neurosurgery Center. Informed consent was obtained from each patient.
Surgical technique
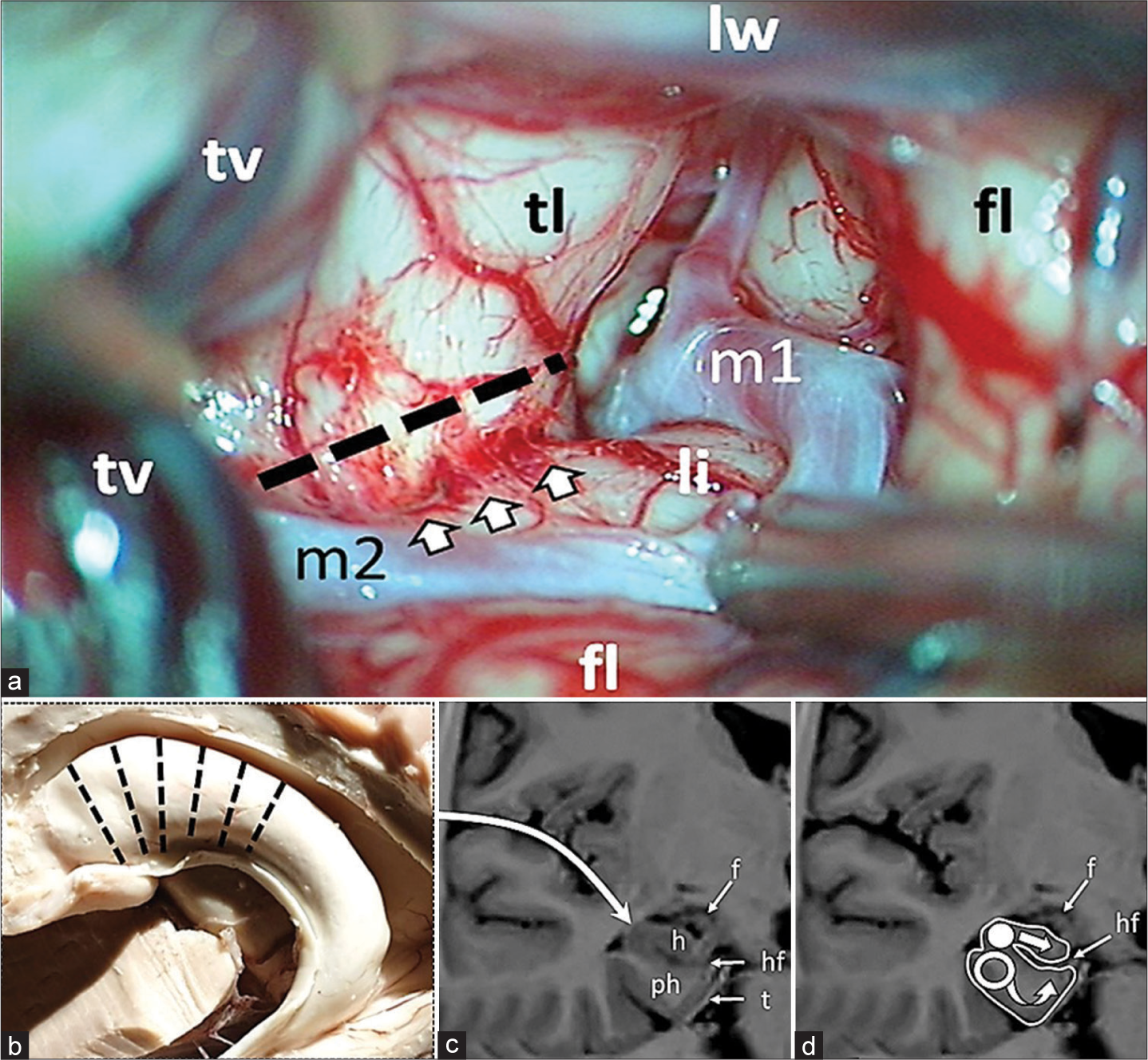
Under general anesthesia with minimal effective dosage of propofol 3–8 mg/kg/h, the patient is placed supine on the operating table with the head fixated on a MAYFIELD skull clamp and tilted about 45° to the opposite site of incision. Standard pterional craniotomy was performed in the left frontotemporal region. After the dura mater was opened an electrode was placed on a basal anterior temporal region and an ECoG was recorded from. The arachnoid of the sylvian fissure was dissected and the sylvian fissure was opened proximally about 3 cm [
Figure 1:
Intraoperative figure and schematic representation of multiple hippocampal transection. (a) Proximal part of the sylvian fissure is opened. The dotted line indicates the site of the temporal stem dissection parallel to the inferior peri-insular sulcus (arrows). fl: Frontal lobe; li: Limen of insule; lw: Lesser wing of sphenoid bone; m1, m2: Segments of middle cerebral artery; tl: Temporal lobe; tv: Temporal vein. (b) Transection locations along hippocampus; (c) Approach is performed through the Sylvian fissure, after lesionectomy and dissection of the limen insula the temporal horn of the lateral ventricle is opened; (d) Hippocampus (h) is intersected in the orthogonal plane to its longitudinal axis with a blunt silver knife (white circle) up to the hippocampal fissure (hf) with preservation of fimbria (f); following transection of hippocampus the parahippocampal gyrus is transected with a blunt silver knife (hollow circle) in the same plane as the hippocampus up to the tentorium (t) and to the arachnoid membrane of the ambient cistern inferomedially and to the hippocampal fissure (hf) dorsally. h: Hippocampus; ph: Parahippocampus; f: Fimbria; hf: Hippocampal fissure; t: Tentorium.
The amygdala was also resected, and the temporal horn of the left lateral ventricle was opened and the anterior part of the head of the hippocampus was exposed. The inferior peri-insular sulcus was identified, and the temporal lobe was dissected along the sulcus at a distance of 15 mm, and thus the surface of the hippocampus, including its tail, was widely exposed. A 4-contact electrode was placed on the surface of the hippocampus from the head to its tail, and ECoG activity was recorded over the hippocampus for 5 min. Registration of characteristic epileptic discharges and spike-wave complexes indicated the involvement of the hippocampus in the generation of seizures, and a decision was made in favor of its transection. Subsequently, the surgery was performed according to the presented scheme [
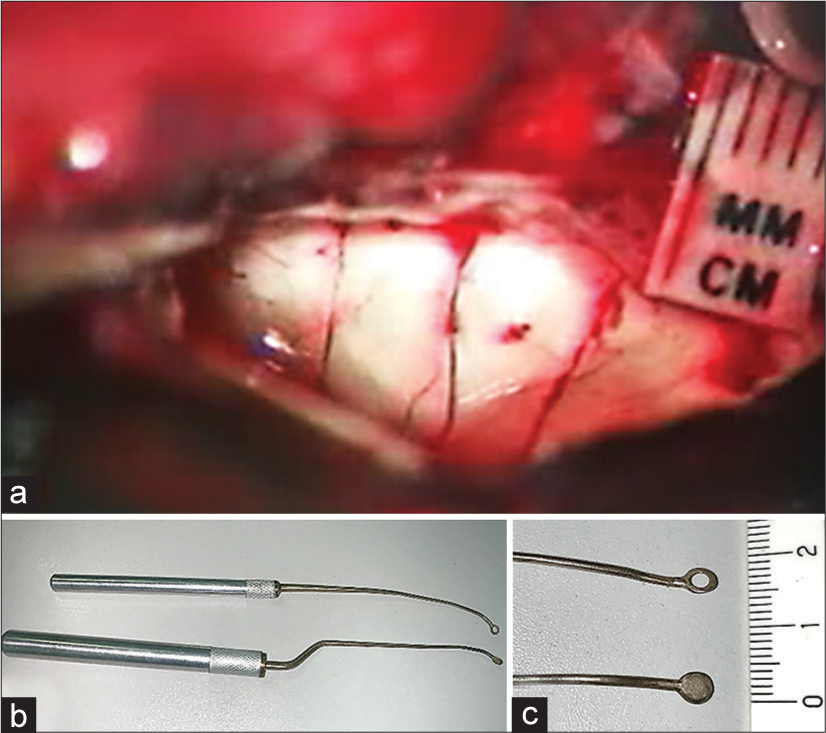
The alveus consisted of dense fibers is sharply incised with micro-scissors. The transverse incision lines are 5 mm apart and perpendicular to the hippocampal axis [
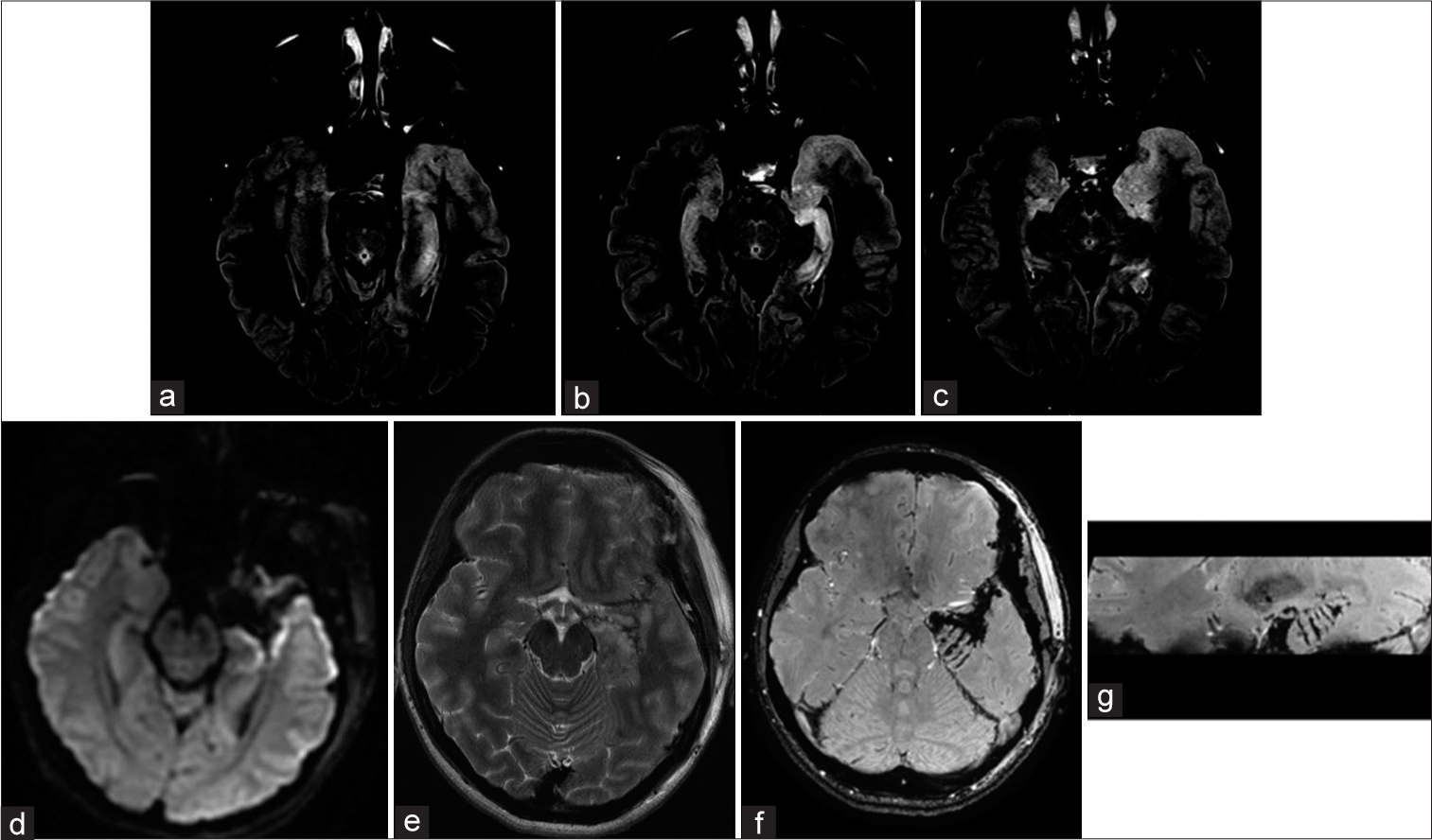
Figure 3:
Multiple hippocampal transections Case #F29. Focal cortical dysplasia, in the left temporal pole is visualized on MRI T2 weighted image. FCD does not spread to the hippocampus and it’s assize and structure were normal. MRI after FCD with the temporal pole resection and hippocampal transection. SWAN, axial and sagittal planes (b and c). Multiple hippocampal transactions. Patient #F27. Preoperative MRI scans on FLAIR reveals focal cortical dysplasia of the temporal pole and uncus. In addition, hippocampal sclerosis without its obvious reduction in volume can be observed on MRI. MRI scans 6 months after resection of the temporal pole, uncus and multiple hippocampal transections. There are no obvious signs of hippocampal infarction on MRI images in DWI (d) and FLAIR (e) sequences; In axial (f) and sagittal (g) MRI images in SWAN sequence, can be clearly visualized transections of hippocampus and parahippocampal gyrus (arrows).
RESULTS
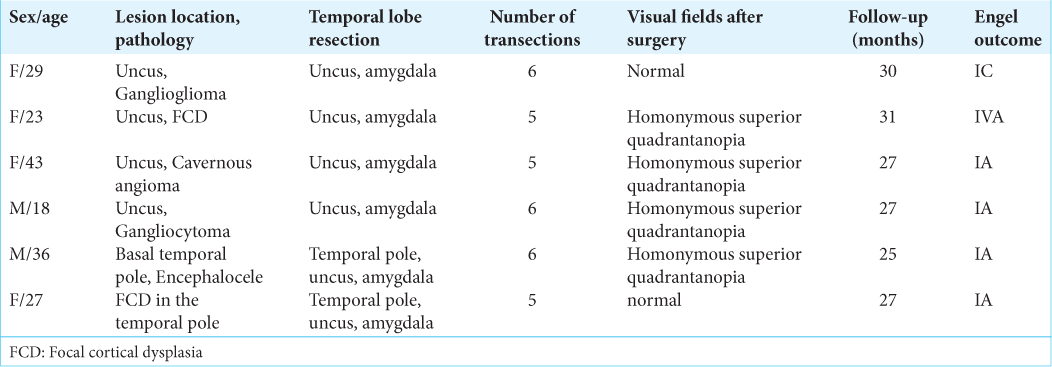
All patients underwent surgery satisfactorily and were discharged from the hospital on average 4 days after surgery. There were no postoperative complications except for homonymous superior quadrantanopia as a consequence of Meyer’s loop injury during temporal lobe stem dissection. This kind of visual field loss was noted in four cases out of six [
Control of postoperative seizures
During the follow-up period of 25–31 months (median 27 months), five patients out of six had Engel Class I outcome (83.3%). In one case, seizures developed after 1 months in a patient with focal cortical dysplasia (FCD) in the uncus (Engel IVA outcome). In the Engel I group, one patient developed rare epileptic seizures 26 months after surgery (Engel IC). During the follow-up period five patients (F29, F23, F43, M18, and M36) are continuing anticonvulsant therapy, and three of these reduced the dose of anticonvulsants (M36, F43, and M18).
Intraoperative ECoG
Despite the resection of lesion, the amygdala, and even the temporal pole in two cases, when recording biopotentials from the hippocampus, sharp wave forms of epileptiform activity were detected in all patients. In two cases (F23 and F27), continued spike activity was recorded. In one patient (M18), activity was minimal, revealed as rare sharp waves.
After hippocampal transections, spikes either completely disappeared in all patients, or single low-amplitude sharp waves were recorded asynchronously under different electrodes (F43). In one case (F23), after hippocampal transections, low-amplitude sharp waves, synchronously conducted from the cortex, were recorded under all electrodes of the strip.
Neuropsychological outcome
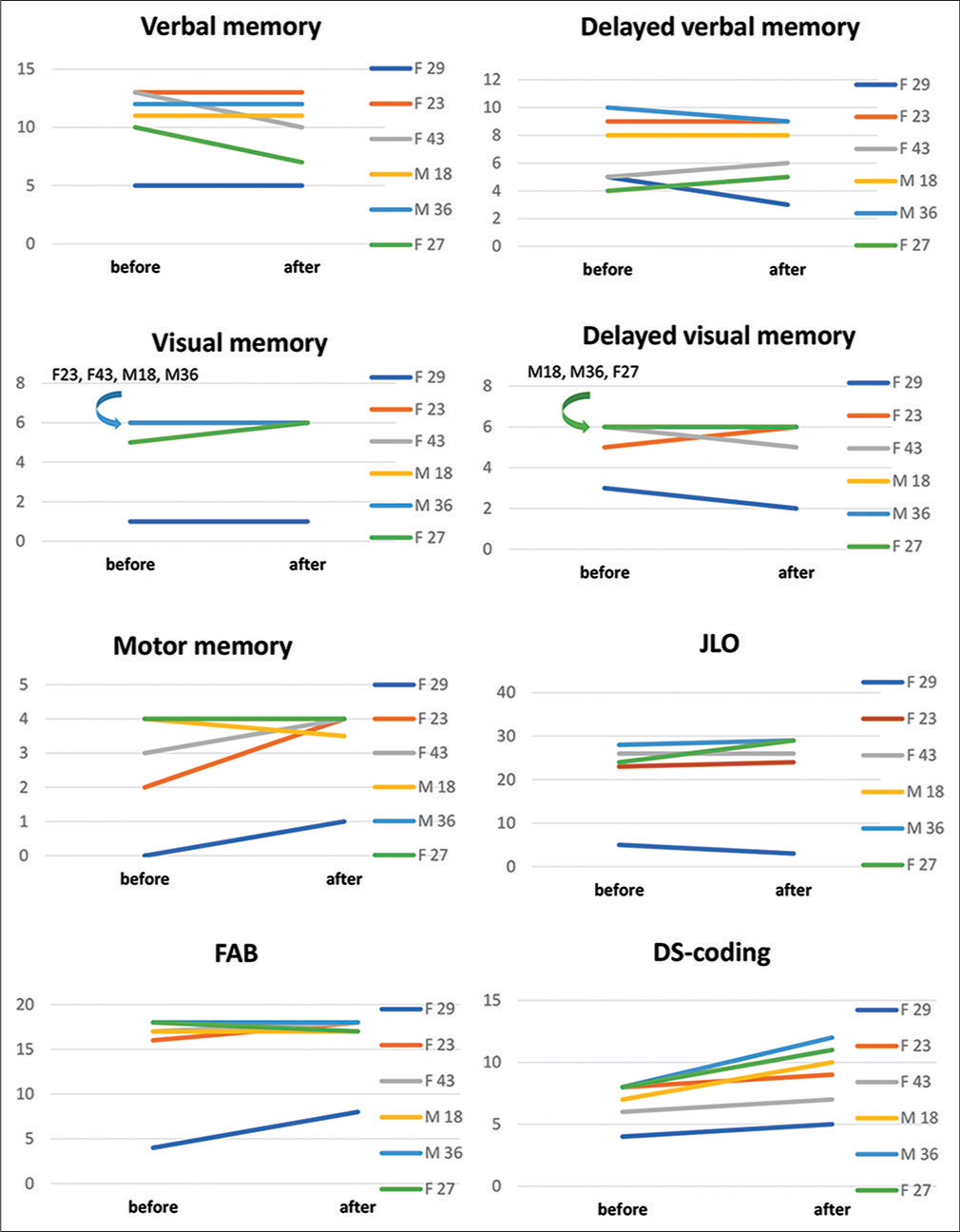
According to results of preoperative neuropsychological evaluation, all patients demonstrated normal indicators on all evaluation scales, except for one patient, F29, where all indicators were decreased.
Immediately after surgery, three out of six patients (M36, F29, and F23) developed significant nominative aphasia which later demonstrated a certain regression; however, they still persisted 6 and 12 months after surgery.
Evaluation of verbal memory in two cases revealed worsening compared to preoperative level [
Delayed verbal memory decreased in two patients (F29 and M36). Improvement was noted in two patients (F43 and F27).
In preoperative period, visual memory was below the normal in one patient (F29). In one case, memory improvement was observed (F27). Delayed visual memory in two cases impaired compared to the preoperative level (F29 and F43).
Preoperative evaluation showed that motor memory was impaired in two patients (F23 and F29); after surgery, one of them showed an improvement relative to preoperative values. In the remaining patients, these indicators remained within the normal range.
When assessing the regulatory component-using block of methods FAB, DS-coding, in the overall preoperative evaluation, regulatory capabilities were impaired in one patient (F29). In post-operative period – relative to preoperative evaluation, three patients showed improvement and three patients remained unchanged.
In the study of visuospatial synthesis, using the JLO technique, only one patient revealed impairment in preoperative evaluation, four patients in postoperative period showed improvement.
DISCUSSION
Significance of MHT and seizure control
Based on recent publications, there is now a consensus that radical excision of neuronal-glial tumors located in the lateral temporal lobe is quite sufficient and hippocampal resection is not necessary for achievement of seizure control.[
The same can be stated about treatment of FCD in the temporal lobe pole; its surgical treatment involves resection of the temporal pole along with the amygdala and hippocampus.[
Considering the above mentioned, it can be stated that surgical resection of the hippocampus contributes to effective treatment and management of seizures. However, excision of a normal functioning hippocampus can lead to serious impairment of verbal memory, visual memory, behavior, and emotions.[
Presurgical predictors of memory impairment after amygdalohippocampectomy are normal architecture and hippocampal volume on MRI scans and normal or slightly reduced memory on neuropsychological testing.[
Milner[
The above stated facts indicate the importance of preserving the hippocampus in cases where the patient’s memory is not affected or is slightly impaired. For this purpose, Shimizu et al.[
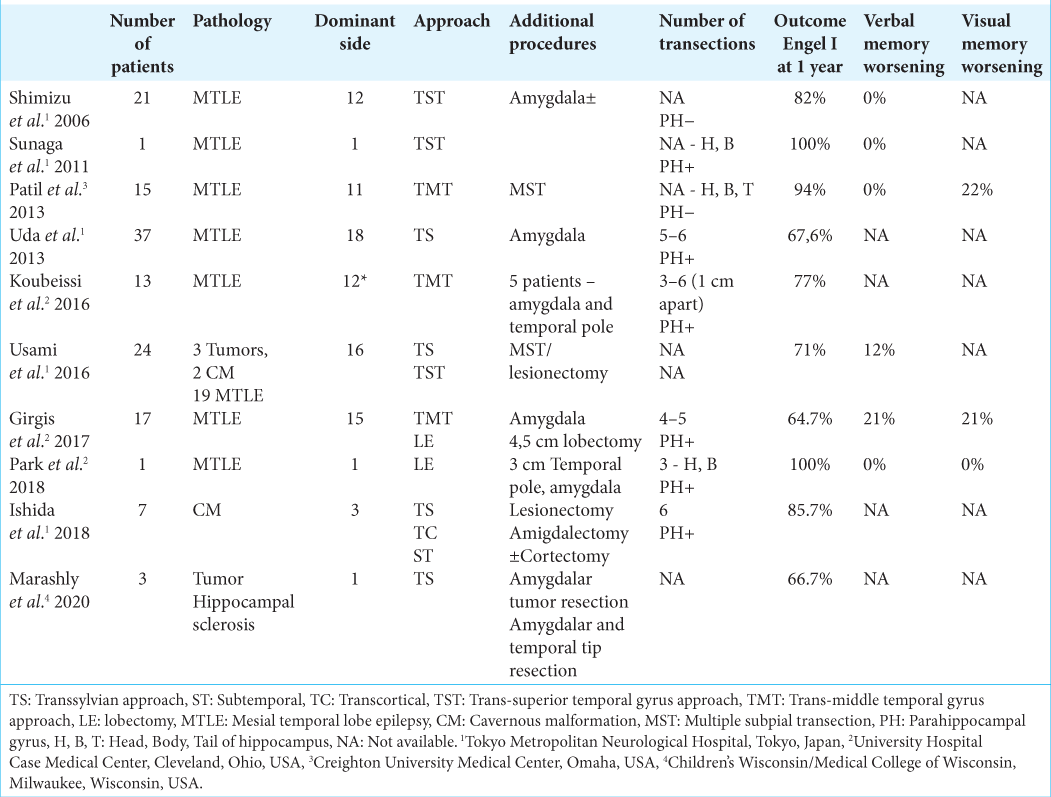
Hippocampal transection was mainly performed in cases of hippocampal sclerosis (126 cases). Only in 13 cases, hippocampal transections were performed for medial temporal space occupying lesions. Of these, cavernous malformations were the cause of epilepsy in nine cases, and neuronal-glial tumors in four cases (three gangliogliomas and 1 DNET).[
In general, taking into account all published data (except for publications describing single observations), after hippocampal transection, seizure control (Engel I) was achieved – from 64.7% to 94.7% of cases during a follow-up period of 12–60 months.[
In our series of patients, four out of six patients had lesions in the medial temporal lobe and two patients in the temporal pole. None of the patients revealed any changes of hippocampus volume or architecture confirmed on MRI scans. After hippocampal transection, an Engel I outcome was achieved in five patients (83.3%) within 27 months after surgery (25–31 months). One patient, with FCD of the temporal pole, developed seizures after 3 months of surgery. In this patient, seizure frequency was much lower than before surgery. Apparently, in this case, the area of initiation of abnormal activity was beyond the temporal lobe, which is not uncommon in FCD.
Neuropsychological outcome
The advantage of MHT lies in its ability to preserve cognitive function, particularly auditory verbal memory on the dominant hemisphere and visuospatial memory on the non-dominant.[
Analysis of few published studies showed that hippocampal transection preserves or even improves verbal and visuospatial memory.[
According to Shimizu et al.,[
According to Ishida et al.,[
In our series, among six patients, two patients relative to the preoperative level demonstrated improvement in delayed verbal memory after MHT. One patient showed a decrease level in delayed verbal memory, and two patients remained unchanged. It is important to note that despite decrease in delayed verbal memory in some patients, it still remains within normal range.
However, it is worth paying attention that in the postoperative period, three patients had nominative difficulties, with only one of them having decreased cognitive abilities in the auditory-verbal link both before and after evaluation. In two other patients with nominative difficulties, auditory-verbal memory indicators remained within the normal range.
This being said, it is important to note that MHT does not impair regulatory abilities, nor does it impair optical-spatial analysis and synthesis, which is a significant indicator of the choice of this type of treatment for patients with initially high cognitive levels.
Limitations
Our study has several important limitations. First, it is a retrospective study with a small number of patients. Second, the outcomes of surgical treatment of different pathologies were evaluated. Third, at the preoperative stage, the patients did not undergo stereo EEG monitoring, which seems to have been the cause of a diagnostic error in patient F29, who suffered from postoperative refractory seizures. Furthermore, the assessment of seizure freedom in the reported patient population cannot be construed as an evaluation of MHT success.
CONCLUSION
MHTs can be considered as an effective method of drug-resistant mesial temporal lobe epilepsy caused by tumors of the medial temporal complex. At the same time, MHT makes it possible to preserve memory in patients with structurally preserved hippocampus. However, MHT do not guarantee the preservation of memory after surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Acsády L, Káli S. Models, structure, function: The transformation of cortical signals in the dentate gyrus. Prog Brain Res. 2007. 163: 577-99
2. Alonso Vanegas MA, Lew SM, Morino M, Sarmento SA. Microsurgical techniques in temporal lobe epilepsy. Epilepsia. 2017. 58: 10-8
3. Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989. 31: 571-91
4. Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl). 2005. 210: 343-52
5. Baxendale S, Thompson P, Kitchen N. Postoperative hippocampal remnant shrinkage and memory decline: A dynamic process. Neurology. 2000. 55: 243-9
6. Dührsen L, Sauvigny T, House PM, Stodieck S, Holst B, Matschke J. Impact of focal cortical dysplasia Type IIIa on seizure outcome following anterior mesial temporal lobe resection for the treatment of epilepsy. J Neurosurg. 2018. 128: 1668-73
7. Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007. 30: 123-52
8. Elliott RE, Bollo RJ, Berliner JL, Silverberg A, Carlson C, Geller EB. Anterior temporal lobectomy with amygdalohippocampectomy for mesial temporal sclerosis: Predictors of long-term seizure control. J Neurosurg. 2013. 119: 261-72
9. Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012. 307: 922-30
10. Engel J. Update on surgical treatment of the epilepsies, Summary of the second international palm desert conference on the surgical treatment of the epilepsies 1992. Neurology. 1993. 43: 1612-7
11. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012. 53: 51-7
12. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012. 70: 921-8
13. Georgiadis I, Kapsalaki EZ, Fountas KN. Temporal lobe resective surgery for medically intractable epilepsy: A review of complications and side effects. Epilepsy Res Treat. 2013. 2013: 752195
14. Girgis F, Greil M, Fastenau P, Sweet J, Lüders H, Miller JP. Resection of temporal neocortex during multiple hippocampal transections for mesial temporal lobe epilepsy does not affect seizure or memory outcome. Oper Neurosurg (Hagerstown). 2017. 13: 711-7
15. Giulioni M, Galassi E, Zucchelli M, Volpi L. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005. 102: 288-93
16. Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: Lesionectomy compared with tailored resection. J Neurosurg. 2009. 111: 1275-82
17. Gleissner U, Helmstaedter C, Schramm J, Elger C. Memory outcome after selective amygdalohippocampectomy: A study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002. 43: 87-95
18. Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003. 54: 425-32
19. Helmstaedter C, Petzold I, Bien CG. The cognitive consequence of resecting nonlesional tissues in epilepsy surgery-results from MRI-and histopathologynegative patients with temporal lobe epilepsy. Epilepsia. 2011. 52: 1402-8
20. Helmstaedter C. Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disord. 2013. 15: 221-39
21. Hermann BP, Wyler AR, Bush AJ, Tabatabai FR. Differential effects of left and right anterior temporal lobectomy on verbal learning and memory performance. Epilepsia. 1992. 33: 289-97
22. Ishida W, Morino M, Matsumoto T, Casaos J, Ramhmdani S, Lo SL. Hippocampal transection plus tumor resection as a novel surgical treatment for temporal lobe epilepsy associated with cerebral cavernous malformations. World Neurosurg. 2018. 119: e209-15
23. Jooma R, Yeh HS, Privitera MD, Rigrish D, Gartner M. Seizure control and extent of mesial temporal resection. Acta Neurochir (Wien). 1995. 133: 44-9
24. Koubeissi MZ, Kahriman E, Fastenau P, Bailey C, Syed T, Amina S. Multiple hippocampal transections for intractable hippocampal epilepsy: Seizure outcome. Epilepsy Behav. 2016. 58: 86-90
25. Labate A, Aguglia U, Tripepi G, Mumoli L, Ferlazzo E, Baggetta R. Long-term outcome of mild mesial temporal lobe epilepsy: A prospective longitudinal cohort study. Neurology. 2016. 86: 1904-10
26. LoGalbo A, Sawrie S, Roth DL, Kuzniecky R, Knowlton R, Faught E. Verbal memory outcome in patients with normal preoperative verbal memory and left mesial temporal sclerosis. Epilepsy Behav. 2005. 6: 337-41
27. Marashly A, Koop J, Loman M, Kim I, Maheshwari M, Lew SM. Multiple hippocampal transections for refractory pediatric mesial temporal lobe epilepsy: Seizure and neuropsychological outcomes. J Neurosurg Pediatr. 2020. 26: 1-10
28. Martin RC, Kretzmer T, Palmer C, Sawrie S, Knowlton R, Faught E. Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Arch Neurol. 2002. 59: 1895-901
29. Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958. 36: 244-57
30. Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005. 28: 223-50
31. Miserocchi A, Cascardo B, Piroddi C, Fuschillo D, Cardinale F, Nobili L. Surgery for temporal lobe epilepsy in children: Relevance of pre-surgical evaluation and analysis of outcome. J Neurosurg Pediatr. 2013. 11: 256-67
32. Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: A new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989. 70: 231-9
33. Patil AA, Andrews R. Long term follow-up after multiple hippocampal transection (MHT). Seizure. 2013. 22: 731-4
34. Patil AA, Chamczuk AJ, Andrews RV. Hippocampal transections for epilepsy. Neurosurg Clin N Am. 2016. 27: 19-25
35. Patil AA, Andrews RV. Nonresective hippocampal surgery for epilepsy. World Neurosurg. 2010. 74: 645-9
36. Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006. 13: 322-8
37. Sindou M, Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguière F. Temporo-mesial epilepsy surgery: Outcome and complications in 100 consecutive adult patients. Acta Neurochir (Wien). 2006. 148: 39-45
38. Tomita T, Volk JM, Shen W, Pundy T. Glioneuronal tumors of cerebral hemisphere in children: Correlation of surgical resection with seizure outcomes and tumor recurrences. Childs Nerv Syst. 2016. 32: 1839-48
39. Trenerry M, Jack CJ, Ivnik R, Sharbrough FW, Cascino GD, Hirschorn KA. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993. 43: 1800-5
40. Uda T, Morino M, Ito H, Minami N, Hosono A, Nagai T. Transsylvian hippocampal transection for mesial temporal lobe epilepsy: Surgical indications, procedure, and postoperative seizure and memory outcomes. J Neurosurg. 2013. 119: 1098-104
41. Umeoka SC, Lüders HO, Turnbull JP, Koubeissi MZ, Maciunas RJ. Requirement of longitudinal synchrony of epileptiform discharges in the hippocampus for seizure generation: A pilot study. J Neurosurg. 2012. 116: 513-24
42. Usami K, Kubota M, Kawai K, Kunii N, Matsuo T, Ibayashi K. Long-term outcome and neuroradiologic changes after multiple hippocampal transection combined with multiple subpial transection or lesionectomy for temporal lobe epilepsy. Epilepsia. 2016. 57: 931-40
43. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345: 311-8
44. Zaghloul KA, Schramm J. Surgical management of glioneuronal tumors with drug-resistant epilepsy. Acta Neurochir (Wien). 2011. 153: 1551-9
45. Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982. 218: 1337-9