- Department of Neurosurgery, National Neuroscience Institute, King Fahed Medical City, Riyadh, Saudi Arabia.
- Department of Emergency Medicine, Ministry of National Guard, King Abdulaziz Medical City, Riyadh, Saudi Arabia.
Correspondence Address:
Mohammed Bafaquh, National Neuroscience Institute, King Fahad Medical City, Riyadh, Saudi Arabia, Riyadh, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_334_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammed Bafaquh1, Abdullah Bahmaid2, Othman T. Almutairi1, Gmaan Alzhrani1, Arwa S. AlShamekh1, Yasser E. Orz1, Mahmoud Alyamany1, Fahd AlSubaie1, Najeeb A. Alomer1, Abdulrahman Y. Alturki1. A potential new brainstem reflex: The oculoglossal phenomenon. 03-Aug-2021;12:388
How to cite this URL: Mohammed Bafaquh1, Abdullah Bahmaid2, Othman T. Almutairi1, Gmaan Alzhrani1, Arwa S. AlShamekh1, Yasser E. Orz1, Mahmoud Alyamany1, Fahd AlSubaie1, Najeeb A. Alomer1, Abdulrahman Y. Alturki1. A potential new brainstem reflex: The oculoglossal phenomenon. 03-Aug-2021;12:388. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11016
Abstract
Background: A synchronized involuntary movement of the tongue to the same side as voluntary movements of the eyes, termed the oculoglossal phenomenon, has been observed. A description of the hypothesized pathway of this phenomenon could guide the development of a rapid clinical evaluation of the long segment of the brainstem and help facilitate further studies to establish a new reflex, if possible. The aim of this study is to describe and propose the simple concept of this pathway/phenomenon, the oculoglossal phenomenon.
Methods: This is an observational study. Of a newly observe brainstem phenomenon evaluated on a subject at the National Neuroscience Institute in king Fahad Medical City (KFMC), Riyadh, Saudi Arabia. After being observed incidentally in a single patient, 60 participants were tested between January and March 2020 to confirm the presence of the phenomenon. Each subject was instructed to protrude the tongue and then move their eyes horizontally to the side. If the tongue simultaneously and involuntarily moved to the same side as the eyes, the test was deemed confirmatory. A literature review was performed, and possible anatomical pathway was proposed.
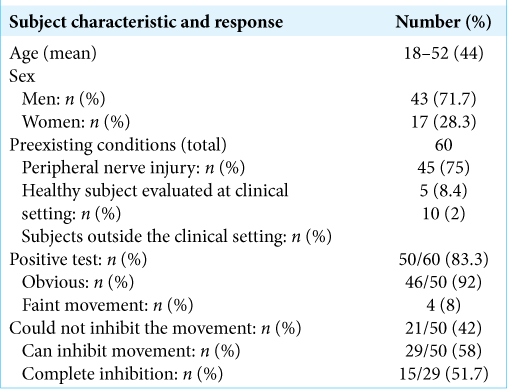
Results: The oculoglossal reflex was present in most (50/60, 83.3%) of the subjects. Our proposed pathway begins at the frontal cortex, followed by a projection to the paramedian pontine reticular formation, then to the contralateral medial longitudinal fasciculus and bilaterally to the hypoglossal nuclei.
Conclusion: An accurate description of this phenomenon could lead to additional studies and possibly establishing it as a legitimate reflex, thus conceivably adding a new tool in the neurological examination to evaluate the brainstem’s integrity.
Keywords: Abducens, Brainstem, Oculomotor, Reflex
INTRODUCTION
Evaluating the cranial nerves and brainstem integrity is essential during a neurological examination, performed either directly or through brainstem reflexes. After Mollaret and Goulon first described brain death and various association reflexes in 1959, brainstem reflexes, their pathways, and their clinical significances have been described, generating a list of brainstem reflexes used by health-care providers to evaluate and monitor the cranial nerves and the brainstem clinically. However, some of these pathways and their clinical significances are not well understood.[
It is crucial to evaluate the tongue and ocular-related reflexes during a neurological examination. Many tongue-related reflexes, such as the extrusion (tongue-thrust) reflex, licking reflexes, and the suck/swallow reflex, have been described in the literature, and while most are part of infant/childhood developmental assessments, few have been described for the adult population.[
We describe a phenomenon that involves both extraocular movement and tongue movement, herein referred to as the oculoglossal phenomenon (OGP). However, we were not able to further test this phenomenon at this stage to evaluate it is specific nature of being a synkinesis or motor overflow of an undescribed pathway that connects the extraocular movement to the tongue movement or a reflex (oculoglossal reflex) that has never been described before. However we are here proposing a potential basic pathway that might explain this novel brainstem reflex (OGR). In a neurosurgical clinic, OGP was observed during a physical examination in which the patient was asked to protrude the tongue for uvular examination. Without instructing the patient to relax the tongue and close his mouth, he was asked to move their eyes horizontally for the extraocular movement test. Interestingly, the tongue immediately and synchronously moved to the same side as the eyes. After this incidental observation, the same instructions were given to other patients, confirming the presence of the OGP. Subsequently, the same set of instructions was used to test a series of subjects (50 patients during routine neurological physical exams) and other 10 healthy people outside the clinical setting.
MATERIALS AND METHODS
After the initial observation of the OGP was made in October 2019, 60 adult subjects mostly patients from the neurosurgical clinic were evaluated for this phenomenon at the National Neuroscience Institute, King Fahd Medical City, between January and March 2020 over 11 sessions. None were known to have brain, brainstem, spine, or cranial nerve pathologies. However, most patients (45) had isolated peripheral nerve injuries without medical comorbidities. The other 15 subjects were healthy and not known to have any illness. All subjects were initially blinded to the specific reason for the test, and as a result, they were not aware of it.
The test procedure steps were to instruct the subjects to protrude the tongue; after 3 seconds, the examiner instructed the patient to move the eyes to the side without moving the head. The test was repeated 3 times with 2 min interval, and each set was performed twice: once at the beginning of the physical examination and again at the end, about 15 min apart. After documenting the findings, the test was repeated with the additional instruction to inhibit tongue movement when moving the eyes. A test was deemed positive if the subjects showed immediate synchronous movement of the tongue when the eyes were moved [
Video 1
PubMed and Google Scholar were searched for reports of this reflex or descriptions of its clinical importance. The search keywords were “ocular,” “oculomotor reflex,” “hypoglossal,” “hypoglossal reflex,” “medial longitudinal fasciculus,” “paramedian pontine reticular formation,” and “oculoglossal phenomenon.”
RESULTS
Of the 60 tested patients, 43 were men, 45 had peripheral nerve injuries, and 15 were healthy and not known to have any illness. In total, 50/60 (83.3%) subjects were deemed to have positive tests, given the apparent synchronous movement between the eyes and tongue to the same side. Of those, 15 (25% of the entire group) could completely inhibit the reflex, 14 (23%) could reduce the movement to some extent, and the remainder were not able to inhibit it [
The literature review revealed no reports describing this phenomenon/reflex or related pathways aside from a few animal studies not directly related to this newly observed phenomenon.[
DISCUSSION
Tongue-related reflexes have been described as primitive infant reflexes in humans and have also been described in primate studies. They are part of the development of infant swallowing and assist in the development of infant feeding mechanisms, which typically disappear as the child grows and begins a regular diet.[
While multiple animal studies have suggested that the hypoglossal nucleus indirectly communicates with the visual system and its related nuclei, no human study has outlined such connection.[
In our study, while most of the participants showed an apparent positive phenomenon, most of the others, who did not show obvious movement of the tongue (7/10, 12% of the entire group), indicated some mouth movement or an urge to move the head. Moreover, due to observing a relative delay in the movement of the tongue following the movement of the eyes, we, therefore, suggest that this phenomenon is probably reflex and less likely to be motor overdrive or synkinesis. We understand that this will need confirmatory EMG and oculography testing and internal and external validation. Finally, we documented that this phenomenon is common and reproducible in a large number of people. Given that many (66%) could inhibit this behavior, a cortical input to its pathway is implied.
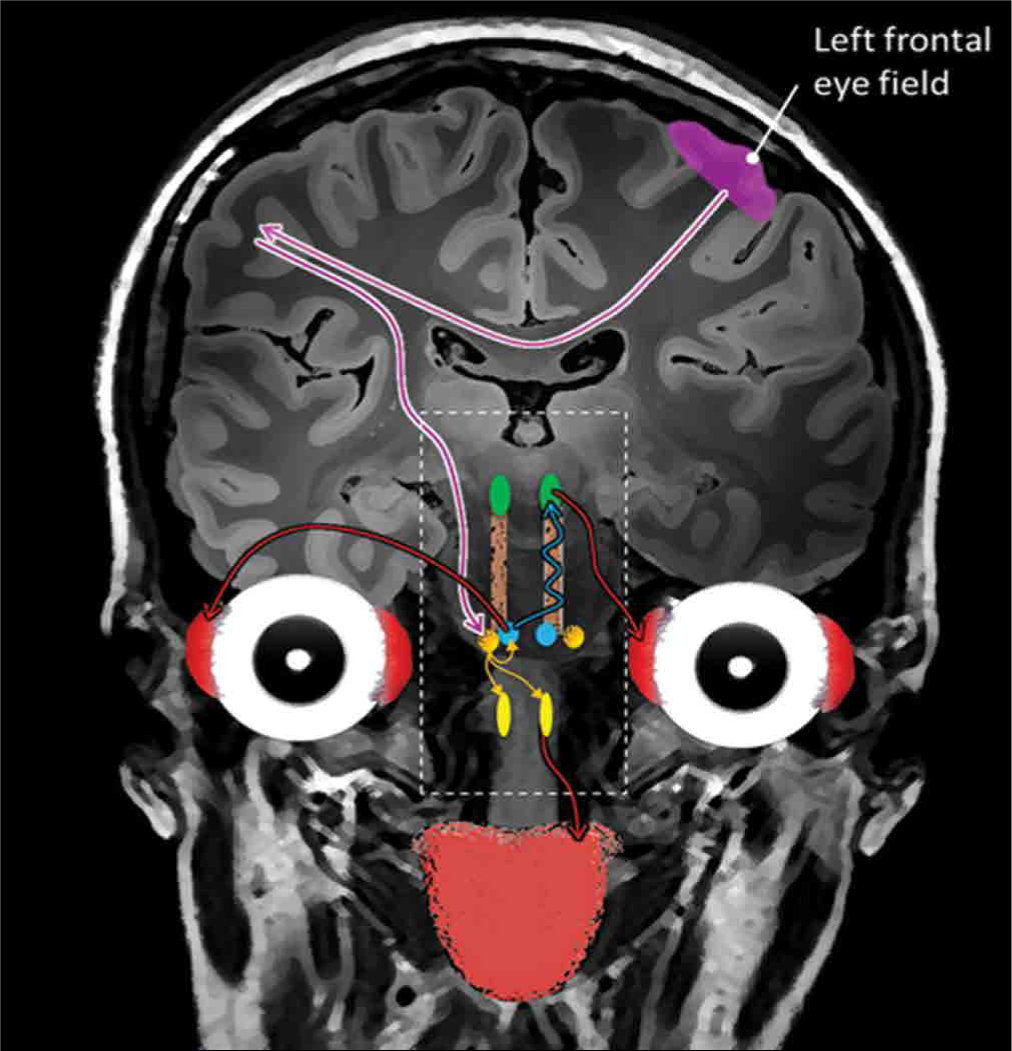
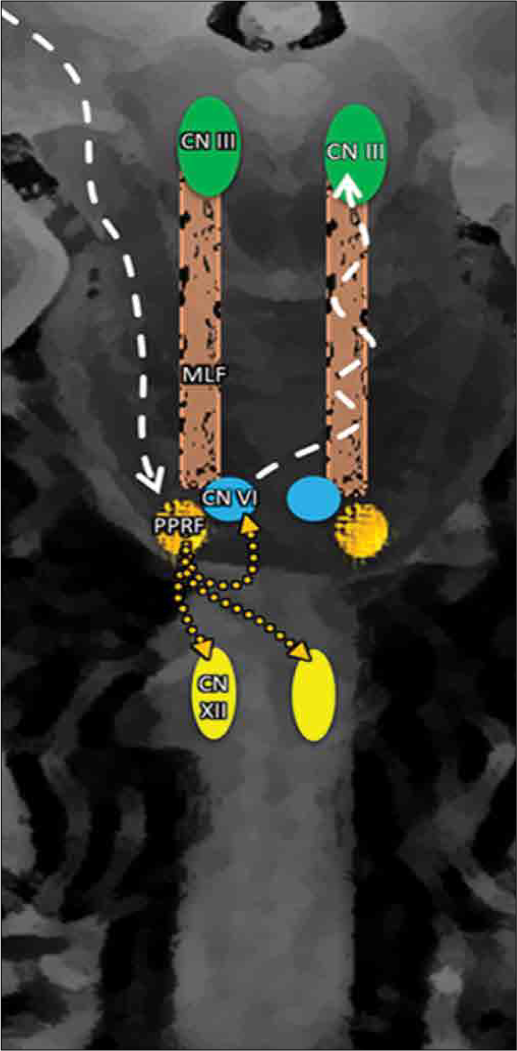
Based on published reports and the similar embryological origins of both extraocular movement nuclei and the hypoglossal nuclei (somites related to the cranial nerve, the somatic motor efferent system), a connectivity of this newly observed phenomenon/reflex can be proposed. The pathway is similar to that of the voluntary horizontal conjugate gaze pathway; the frontal eye fields initiate it, directly and indirectly, then it projecting to the contralateral paramedian pontine reticular formation (PPRF). We propose that the PPRF connects to the ipsilateral CN VI directly, contralateral CN III through the MLF, and bilaterally connects to the hypoglossal nuclei (HMN) to inhibit the ipsilateral hypoglossal nucleus, and activating the contralateral hypoglossal nucleus. This pathway and connection lead to the synchronization of tongue movement and horizontal eye movements to the same side through the known MLF pathway activation [
Figure 1:
Overview of the proposed oculoglossal reflex pathway: Cortical activation is in the frontal eye field, which projects directly and indirectly to the paramedian pontian reticular formation, which then sends fibers to the abducens nerve (CN VI) and the bilateral hypoglossal nuclei. From the CN VI, fibers will project to the oculomotor nucleus (CN III). This pathway leads to contraction of the contralateral lateral rectus muscle, ipsilateral medial rectus muscle, and ipsilateral extrinsic and intrinsic tongue muscles, which leads to synchronized movements of the eyes and tongue to the right side (contralateral).
We believe that this phenomenon provides a potential clinical application. It might allow us to evaluate the integrity of the long segment of the brainstem, including the midbrain (CN III), the pons (PPRF and CN VI), and the medulla (CN XII), to be easily tested.
Limitation
The primary limitations of this study are the lack of detailed neurophysiological and experimental evaluations to elucidate the connection and pathway and identify the nature of this phenomena being one of three; reflex, synkinesis, or motor overflow. In addition, we selected a very specific group of subjects to confirm the reflex’s presence without validation.
CONCLUSION
This newly observed brainstem phenomenon could potentially be a vital tool in the neurological examination. Our proposed anatomical pathway, if proven correct, would help evaluate a long segment of the brainstem in a simple clinical way.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on:
www.surgicalneurologyint.com
References
1. Arvedson JC.editors. Swallowing and Feeding in Infants and Young Children. GI Motility Online. 2006. p.
2. Buttner U, Buttner-Ennever JA. Present concepts of oculomotor organization. Prog Brain Res. 2006. 151: 1-42
3. Elmundt J, Bowman JP, Morgan RJ. Vestibular influence on tongue activity. Exp Neurol. 1983. 18: 126-40
4. Frohman TC, Galetta S, Fox R, Solomon D, Straumann D, Filippi M. Pearls and Oy-sters: The medial longitudinal fasciculus in ocular motor physiology. Neurology. 2008. 70: e57-67
5. Guo H, Yuan XS, Zhou JC, Chen H, Li SQ, Qu WM. Whole-brain monosynaptic inputs to hypoglossal motor neurons in mice. Neurosci Bull. 2020. 36: 585-97
6. Hentschel J, Ruff R, Juette F, von Gontard A, Gortner L. Neonatal facial movements in the first minutes of life-eye opening and tongue thrust: An observational study. Am J Perinatol. 2007. 24: 611-8
7. Mameli O, Tolu E. Electromyographic activity of the tongue muscles during photic stimulation of the retina. IRCS Med Sci. 1985. 13: 44-450
8. Mameli O, Tolu E. Responses of hypoglossal neurons to vestibular stimulation. Neurosci Lett Suppl. 1984. 18: 81
9. Matis G, Chrysou O, Silva D, Birblis T. Brain death: History, updated guidelines and unanswered questions. Internet J Neurosurg. 2012. 8: 1-3
10. Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: Their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002. 13: 409-25
11. Mollaret P, Goulon M. The depassed coma (preliminary memoir). Rev Neurol (Paris). 1959. 101: 3-15
12. Okamoto R, Saito Y, Inoue T, Maegaki Y, Nagaishi J, Ohno K. Forced mouth opening reaction: A primitive reflex released from cortical inhibition. Brain Dev. 2006. 28: 272-4
13. Sauerland EK, Mitchel SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med. 1975. 33: 445-55
14. Scudder CA, Kaneko CRS, Fuchs AF. The brainstem burst generator for saccadic eye movements. A modern synthesis. Exp Brain Res. 2002. 142: 439-62
15. Yap SM, Iyer P, Smyth S. Reflex tongue protrusion as a novel release phenomenon in dementia in adulthood. Mov Disord Clin Pract. 2018. 5: 661-2