- Department of Neurosurgery, Cantonal Hospital St. Gallen, Switzerland.
- Department of Orthopedics and Traumatology Cantonal Hospital St. Gallen, Switzerland.
- Department of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St. Gallen, Switzerland.
Correspondence Address:
Michal Ziga, Department of Neurosurgery, Cantonal Hospital St. Gallen, Switzerland.
DOI:10.25259/SNI_769_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michal Ziga1, Daniele Gianoli2, Frederike Waldeck3, Cyrill Dennler2, Rainer Schlichtherle2, Thomas Forster2, Benjamin Martens2, Roman Schwizer2. Spondylodiscitis due to anaerobic bacteria Veillonella parvula: Case report and literature review. 30-Sep-2021;12:496
How to cite this URL: Michal Ziga1, Daniele Gianoli2, Frederike Waldeck3, Cyrill Dennler2, Rainer Schlichtherle2, Thomas Forster2, Benjamin Martens2, Roman Schwizer2. Spondylodiscitis due to anaerobic bacteria Veillonella parvula: Case report and literature review. 30-Sep-2021;12:496. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11143
Abstract
Background: While pyogenic spondylodiscitis due to Gram-positive aerobic bacteria and its treatment is well known, spondylodiscitis caused by anaerobic Gram-negative pathogen is rare. In particular, the spondylodiscitis caused by Veillonella species is an absolute rarity. Thus no established management recommendations exist.
Case Description: A case report of a 79-year-old man with spondylodiscitis caused by Veillonella parvula with intramuscular abscess collection managed conservatively with stand-alone antibiotic therapy without a spinal stabilization procedure. A review of literature of all reported spondylodiscitis caused by Veillonella species was performed. After 3 week-intravenous therapy with the ceftriaxone in combination with the metronidazole followed by 3 weeks per oral therapy with amoxicillin/clavulanate, the complete recovery of the patient with the V. parvula infection was achieved.
Conclusion: Treatment of the spondylodiscitis caused by Veillonella species should contain a beta-lactam with beta-lactamase inhibitor or third-generation cephalosporine. Six weeks of treatment seem to be sufficient for the complete recovery of the patient.
Keywords: Infection, Low back pain, Spine, Spondylodiscitis, Veillonella
INTRODUCTION
Spondylodiscitis, a term used for vertebral osteomyelitis, spondylitis, and discitis, is a severe disease, which occurs in patients with increasing age and risk factors such as diabetes, immunodeficiency, malignancy, intravenous drug use, recent gastrointestinal procedures, dental diseases/procedures, and renal failure. They can be causative to severe events such as meningitis,[
Veillonella species are small, non-motile, non-fermentative, strictly anaerobic, Gram-negative cocci. They form part of the normal flora of the gastrointestinal tract, urogenital tract, and respiratory tract. Veillonella uses only a few glucose metabolites, especially lactic acid for energy production/ consumption. That is why the strictly anaerobic conditions are essential for maintaining the growth of the bacterium.[
Spondylodiscitis is typically managed with antibiotics. Surgical treatment is indicated in the presence of implantat-associated disease, neurological symptoms, intraspinal and epidural abscesses, signs of spinal instability, or refractory pain.[
We present a rare case of a spondylodiscitis with intramuscular abscess collection in the psoas due to anaerobic pathogen – V. parvula.
We performed a search via PubMed using the search terms “Veillonella,” “Spondylodiscitis,” “vertebral/spinal osteomyelitis” and “spinal infection” to reveal all publications on spondylodiscitis with Veillonella species. Until this date, only ten cases of spinal infection caused by Veillonella species have been published.
CASE DESCRIPTION
A 79-year-old man presented with a 1-month history of constant lower back pain (LBP) radiating to the right thigh. The pain had been increasing over time. Movements aggravated the symptoms. The patient reported 10 out of 10 points in the visual analog scale. He was afebrile but reported intermittent chills at night. There was no history of recent gastrointestinal procedures, dental diseases/procedures, oncological diseases, immunodeficiency, diabetes, or intravenous drug use.
On examination, the lumbar paravertebral muscles were tender, hip flexion on the right side revealed slightly paresis (M4/5), and minimal movement of the spine or the limbs was accompanied by severe pain.
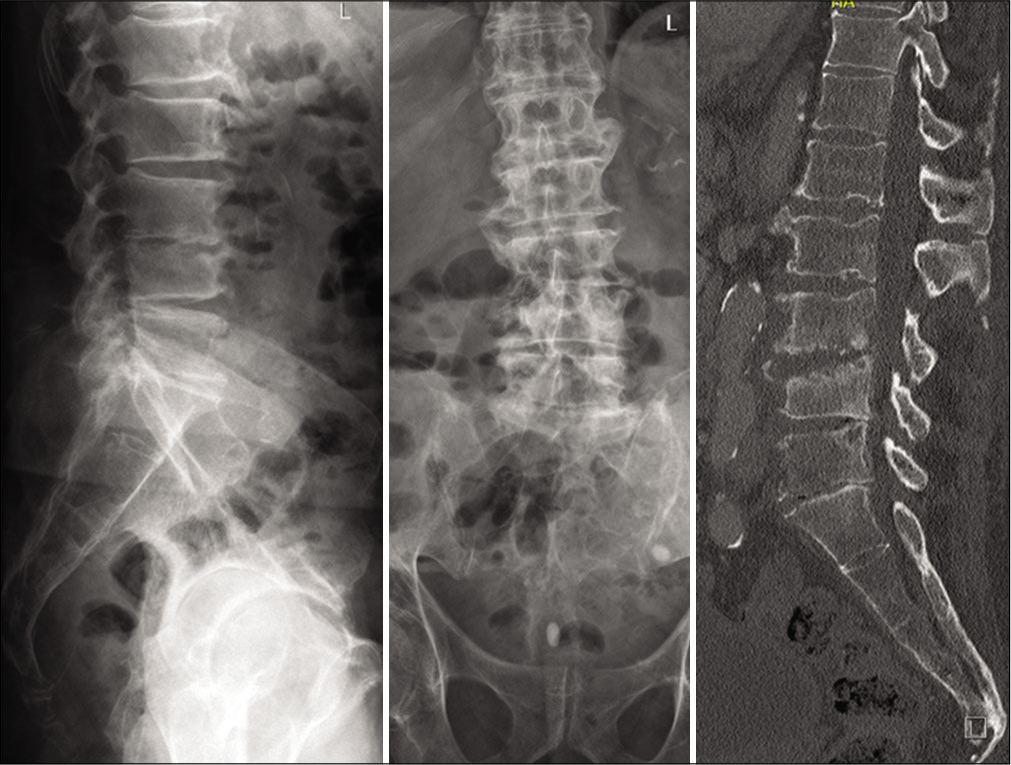
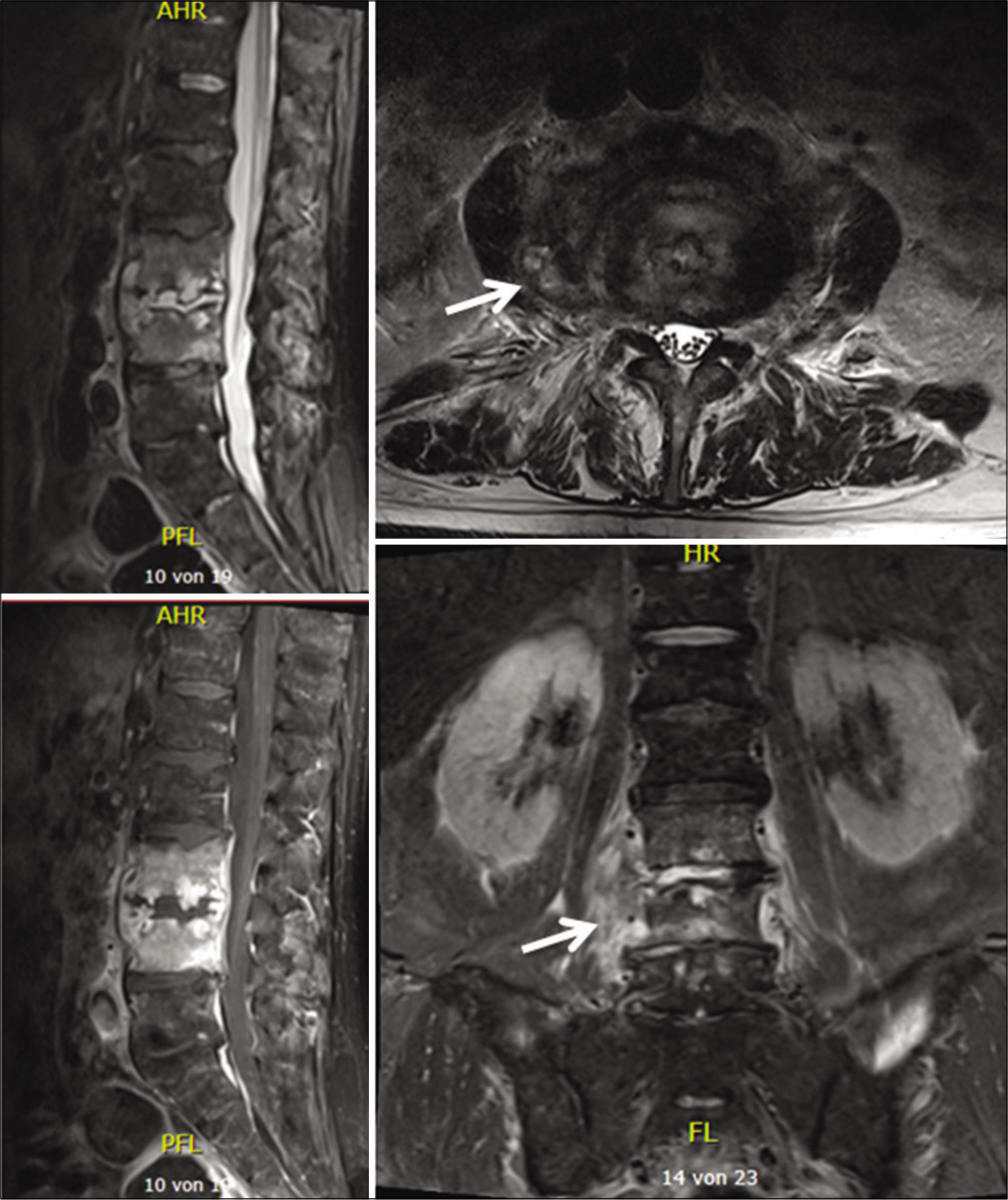
On admission, the inflammation markers were elevated (C-reactive protein [CRP] 54,4 mg/l, white blood cells [WBC] count = 13,6 G/l, platelet count = 344 G/l). X-ray and a ct-scan of the lumbar spine revealed destruction of the intervertebral space L3/4 and the corresponding endplates. Because of the bony destruction, the L4-body presented an anterior wedging. The standing X-ray revealed a segmental kyphosis as a result of the wedge deformity of L4-body and the collapsed disc space L3/4 [
Figure 1:
An X-ray/CT scan of the lumbar spine revealed the destructive pathological changes in the intervertebral space as well as in the adjacent plates of the L3 and L4 (ventrally>dorsally) resulting in the segmental kyphosis in the segment L3/4 on the lateral view. The loss of the disc space L3/4, especially on the right side could be seen in the anteroposterior scans/radiographs.
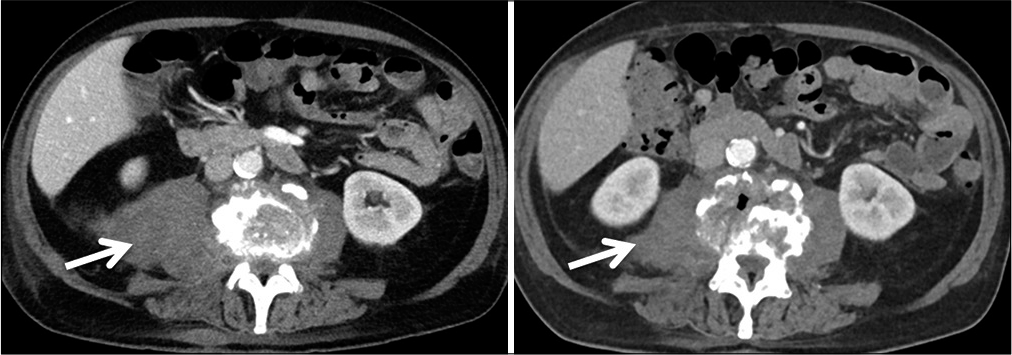
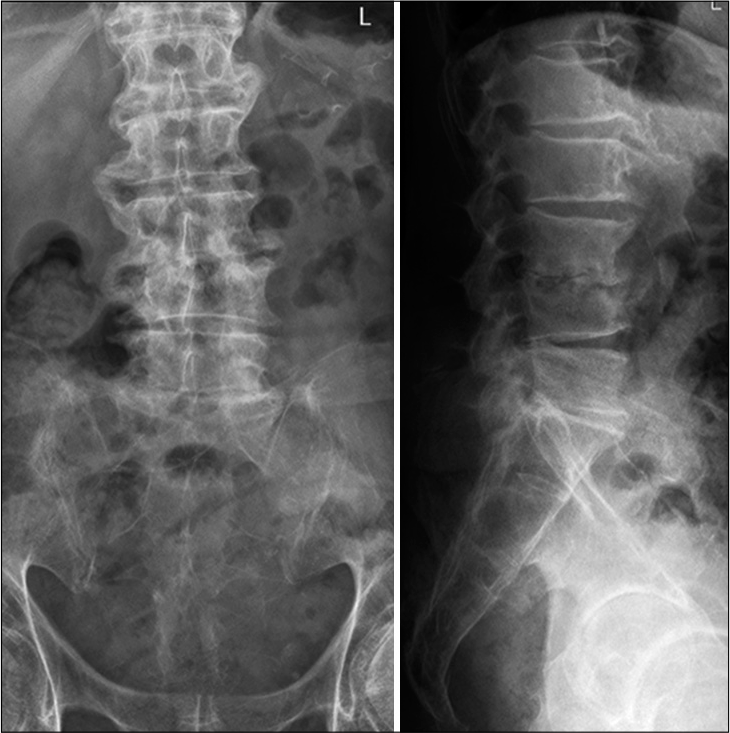
Abnormal high calcium levels revealed an incidental parathyroid adenoma, which had to be removed surgically. Because of the additional surgery, the patient was discharged on day 27. On day 39, the patient presented himself in good health and pain-free to our outpatient clinic. Inflammation markers had normalized (CRP 2 mg/l, normal WBC count). A small residual abscess collection in the right psoas muscle was discovered on CT scan. The CT-scans and the standing X-rays of the lumbar spine showed no instability of the spine without any progression of the kyphotic deformity L3/L4. The antibiotics were switched to amoxicillin/clavulanate 1 g po 3 times daily for another 2 weeks, completing 6 weeks of the antibiotic treatment altogether. The patient presented himself to the last follow-up at day 95 in perfect shape, without any symptoms, and further changes in the lumbar spine X-rays [
There was no external immobilization of the spine during the whole duration of the treatment.
RESULTS
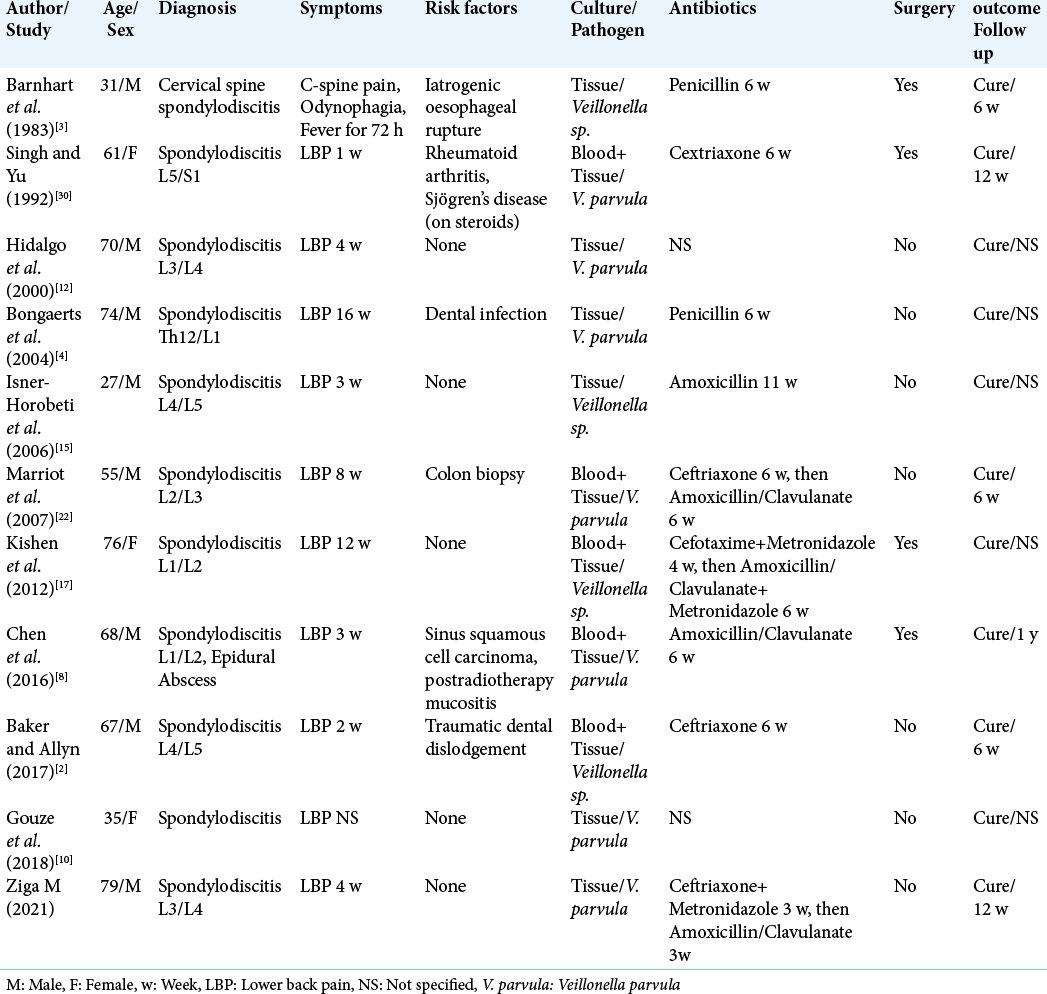
Cases of spondylodiscitis caused by Veillonella species are summarized in [
The first to report Veillonella spondylodiscitis was in 1983 Barnhart et al.[
Singh and Yu[
Another case presented by Kishen et al.[
In the previous four cases, surgical therapy had to be performed additionally to antibiotic treatment.
In the next six cases, stand-alone antibiotic therapy was sufficient to cure the patients completely.
Bongaerts et al.[
A source of infection could be identified in the previous three cases: In the first case, poor dental status with periodontitis was considered the entry point. In the second case, the colonoscopy with the rectal biopsy was suspected as the cause, and in the third case, it was traumatic dental dislodgement. On the other hand, three cases of healthy patients without the risk factors suffering the Veillonella spondylodiscitis have also been published. Isner-Horobeti et al. published the first such case.[
In five cases out of ten known in literature, the length of antibiotic therapy was 6 weeks. In other three cases, the length of the therapy was 11 or 12 weeks. In two cases, the length of the therapy was not specified. In all ten cases, the beta-lactam antibiotics in different grades were used, in one case in combination with the metronidazole.
DISCUSSION
We report a severe case of spondylodiscitis and abscess of the psoas muscle caused by V. parvula. To the best of our knowledge, this rare anaerobic pathogen has only been reported in ten further cases previously [
Once detected, the Veillonella species are mostly part of the polymicrobial processes or/and are often considered contamination and non-pathogenic.[
As the Veillonella grows slowly and under strictly anaerobic conditions, it can take 4–5 days until diagnosis and antibiotic resistance is done. Due to the lack of literature, there is no specific antibiotic strategy to treat the infection.
Veillonella species respond well to penicillin therapy and in vitro to cephalosporins, ceftriaxone, clindamycin, metronidazole, and chloramphenicol. On the other hand, the resistance was shown to vancomycin, tetracycline, aminoglycoside, and ciprofloxacin.[
Our patient was cured with a 6-week antibiotic therapy with beta-lactams. (Ceftriaxone+Metronidazol for 3 weeks, Amoxicillin/Clavulanate for 3 weeks). The same therapy approach was also reported by others. Thus, based on case reports and our experience we recommend treatment of spondylodiscitis caused by Veillonella species with betalactame plus beta-lactamase-inhibitor for 6 weeks.
The primary source of V. parvula in the presented case remains unclear as the patient had no risk factors or interventions performed (good dental status, no diabetes, good renal functions). As Veillonella is a pathogen from the gastrointestinal tract, source of seeding is suggested to be from the oral cavity or intestine. We advice on performing radiographs of the abdomen (CT-scan and colonoscopy) and visitation of a dentist to rule out malignancy other abscesses.
Even though this was also performed in our patient primary source could not be identified.
CONCLUSION
We present a case of spondylodiscitis caused by V. parvula with good clinical outcome. Surprisingly no risk factors and predispositions such as instrumentation or diseases of the digestive tract could be identified. We advise physicians to maintain a high level of suspicion when Veillonella is discovered on cultures or by PCR from biopsies. Treatment should contain a beta-lactam with beta-lactamase inhibitor or third-generation cephalosporine. Six weeks of treatment seem to be sufficient for the complete recovery of the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. Incidence and risk factors for mortality of vertebral osteomyelitis: A retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013. 3: e002412
2. Baker S, Allyn R. Lytic lesions: Looking lethal but leaving room for a simple cure? A case of Veillonella spinal osteomyelitis. JMM Case Rep. 2017. 4: e005108
3. Barnhart RA, Weitekamp MR, Aber RC. Osteomyelitis caused by Veillonella. Am J Med. 1983. 74: 902-4
4. Bongaerts GP, Schreurs BW, Lunel FV, Lemmens JA, Pruszynski M, Merkx MA. Was isolation of Veillonella from spinal osteomyelitis possible due to poor tissue perfusion?. Med Hypotheses. 2004. 63: 659-61
5. Brook I. Veillonella infections in children. J Clin Microbiol. 1996. 34: 1283-5
6. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997. 79: 874-80
7. Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007. 16: 1307-16
8. Chen YC, Ko PH, Yang CJ, Chen YC, Lay CJ, Tsai CC. Epidural abscess caused by Veillonella parvula Case report and review of the literature. J Microbiol Immunol Infect. 2016. 49: 804-8
9. Delwiche EA, Pestka JJ, Tortello ML. The Veillonellae Gram negative cocci with a unique physiology. Annu Rev Microbiol. 1985. 39: 175-93
10. Gouze H, Noussair L, Padovano I, Salomon E, de Laroche M, Duran C. Veillonella parvula spondylodiscitis. Med Mal Infect. 2019. 49: 54-8
11. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000. 25: 1668-79
12. Hidalgo C, Piedrola G, Guzman M, Jimenenez-Alfonso JF. Backache in a 70-year old man. Enferm Infecc Microbiol Clin. 2000. 18: 241-2
13. Hirai J, Yamagishi Y, Kinjo T, Hagihara M, Sakanashi D. Osteomyelitis caused by Veillonella species: Case report and review of the literature. J Infect Chemother. 2016. 22: 417-20
14. Hughes CV, Kolenbrander PE, Andersen RN, Moore LV. Coaggregation properties of human oral Veillonella spp. : Relationship to colonization site and oral ecology Appl Environ Microbiol. 1988. 54: 1957-63
15. Isner-Horobeti M, Lecocq J, Dupeyron A, de Martino SJ, Froehlig P, Vautravers P. Veillonella discitis. A case report. Joint Bone Spine. 2005. 73: 113-5
16. Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: A 14-year population-based study. J Infect. 2014. 68: 313-20
17. Kishen TJ, Lindstrom ST, Etherington G, Diwan AD. Veillonella spondylodiscitis in a healthy 76-year-old lady. Eur Spine J. 2012. 21: 413-7
18. Liaw YS, Yang PC, Wu ZG, Yu CJ, Chang DB, Lee LN. The bacteriology of obstructive pneumonitis. A prospective study using ultrasound-guided transthoracic needle aspiration. Am J Respir Crit Care Med. 1994. 149: 1648-53
19. Liu JW, Wu JJ, Wang LR, Teng LJ, Huang TC. Two fatal cases of Veillonella bacteremia. Eur J Clin Microbiol Infect Dis. 1998. 17: 62-4
20. Loughrey AC, Chew EW. Endocarditis caused by Veillonella dispar. J Infect. 1990. 21: 319-21
21. Marchandin H, Teyssier C, Carriere C, Canovas F, Daras H, Jumas-Bilak E. Prosthetic joint infection due to Veillonella dispar. Eur J Clin Microbiol Infect Dis. 2001. 20: 340-2
22. Marriott D, Stark D, Harkness J. Veillonella parvula discitis and secondary bacteremia: A rare infection complicating endoscopy and colonoscopy?. J Clin Microbiol. 2007. 45: 672-4
23. Martin WJ, Gardner M, Washington JA. In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972. 1: 148-58
24. Nukina S, Hibi A, Mishida K. Bacterial meningitis caused by Veillonella parvula. Acta Paediatr Jpn. 1989. 31: 609-14
25. Nyfors S, Kononen E, Bryk A, Syrjanen R, JousimiesSomer H. Age-related frequency of penicillin resistance of oral Veillonella. Diagn Microbiol Infect Dis. 2003. 46: 279-83
26. Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999. 44: 1018-25
27. Rolfe RD, Finegold SM. Comparative in vitro activity of new beta-lactam antibiotics against anaerobic bacteria. Antimicrob Agents Chemother. 1981. 20: 600-9
28. Rolfe RD, Finegold SM. Comparative in vitro activity of ceftriaxone against anaerobic bacteria. Antimicrob Agents Chemother. 1982. 22: 338-41
29. Sato T, Matsuyama J, Sato M, Hoshino E. Differentiation of Veillonella atypica, Veillonella dispar and Veillonella parvula using restricted fragment-length polymorphism analysis of 16S rDNA amplified by polymerase chain reaction. Oral Microbiol Immunol. 1997. 12: 350-3
30. Singh N, Yu VL. Osteomyelitis due to Veillonella parvula Case report and review. Clin Infect Dis. 1992. 14: 361-3
31. Sutter VL, Finegold SM. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976. 10: 736-52
32. Warner JF, Perkins RL, Cordero L. Metronidazole therapy of anaerobic bacteremia, meningitis, and brain abscess. Arch Intern Med. 1979. 139: 167-9