- Department of Neurosurgery, Division of Pediatric Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
Correspondence Address:
Tanya Minasian, DO, Department of Neurosurgery, Division of Pediatric Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
DOI:10.25259/SNI_194_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Taylor Anne Wilson, Vadim Gospodarev, Sean Hendrix, Tanya Minasian. Pediatric abusive head trauma: ThinkFirst national injury prevention foundation. 19-Oct-2021;12:526
How to cite this URL: Taylor Anne Wilson, Vadim Gospodarev, Sean Hendrix, Tanya Minasian. Pediatric abusive head trauma: ThinkFirst national injury prevention foundation. 19-Oct-2021;12:526. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11186
Abstract
Background: Pediatric abusive head trauma (AHT) represents 80% of nonaccidental trauma deaths, remaining a lead cause of death among infants and young children. Furthermore, neurosurgical intervention can ameliorate damage from secondary injury, but we are currently unable to alter the impact of the primary injury. Thus, prevention through increased public awareness is imperative. This study identifies injuries and predictors of outcomes in pediatric AHT and highlights the importance of partnering with our community through ThinkFirst, a national injury prevention foundation, to educate parents and caregivers about prevention.
Methods: This single-institution retrospective review identifies injuries and predictors of outcomes in pediatric AHT and highlights the importance of partnering with our community to raise awareness and educate parents and caregivers about prevention.
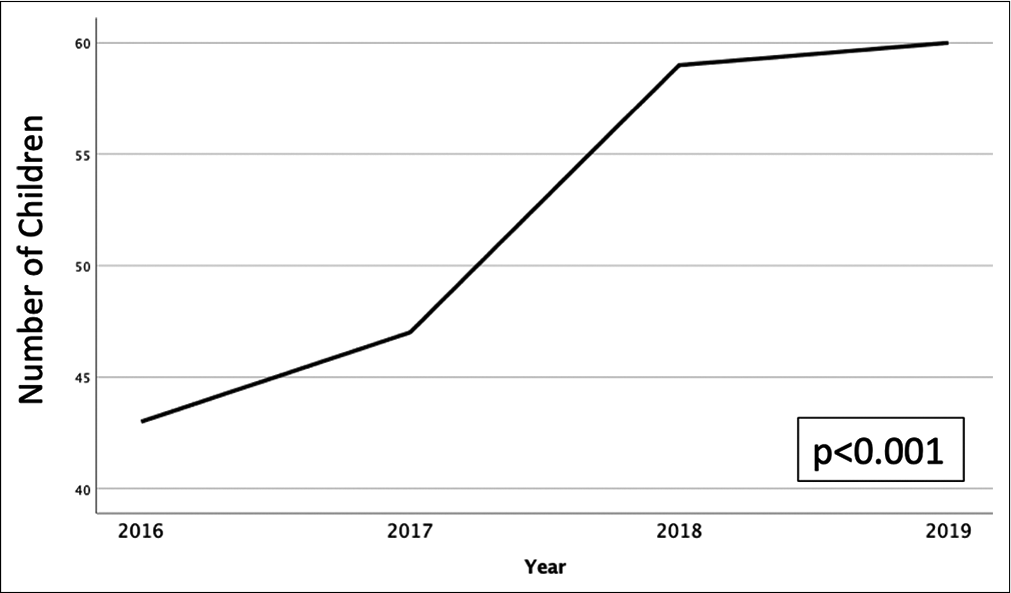
Results: The number of pediatric AHT cases continues to steadily increase over time (P P
Conclusion: The population of patients with AHT is unique, and one that will benefit from continued efforts at increased multidisciplinary and public awareness. Prevention of AHT through awareness is critical. Through partnering with ThinkFirst, a national injury prevention foundation, we aim to educate parents and caregivers about prevention.
Keywords: Abusive head trauma, Nonaccidental trauma, Pediatric, ThinkFirst
INTRODUCTION
Pediatric abusive head trauma (AHT) is defined by the Centers for Disease Control as “an injury to the skull or intracranial contents of an infant or child younger than 5 years caused by inflicted blunt impact, violent shaking, or both.”[
Neurosurgical intervention can ameliorate damage from secondary injury, but we are currently unable to alter the impact of the primary injury. Thus, prevention through increased public awareness is imperative. This study identifies injuries and predictors of outcomes in pediatric AHT and highlights the importance of partnering with our community, in a region where rates of AHT are increasing exponentially, to raise awareness and educate parents and caregivers about prevention.
MATERIALS AND METHODS
This is a single institution, retrospective study of patients suffering AHT from January 1, 2016, to December 31, 2019. All pediatric patients age 4 and younger who presented to our institution with AHT during this time were included in the study. Patients were obtained through the Trauma Registry. As it can be notoriously difficult to delineate between the two, our hospital’s forensics team investigates each possible NAT at the time of presentation and determines whether accidental or nonaccidental events occurred. To further ensure only patients who were victims of NAT were included in the study, all patients obtained from the Trauma Registry were then verified by the authors as NAT. Patients with head trauma that was not determined to be abusive or nonaccidental in nature were excluded from this study. We obtained a waiver of consent for this retrospective institutional study.
Data collected and analyzed included demographic information, injury information, treatment information, and outcomes measures. Outcomes measured included morbidity and mortality data, length of stay, disposition, and cost of hospitalization. A subgroup analysis was also performed on patients with intracranial hemorrhage or injury, excluding those patients with only skull fractures. Payer information was also included. Data were analyzed using SPSS Statistics for Macintosh, Version 26.0 (Armonk, NY: IBM Corp., 2019). The Kolmogorov–Smirnov test for normality was performed, and since data were not normally distributed, nonparametric statistical tests were used. Univariate analysis was performed using the Spearman’s Rho, Mann–Whitney U, Kruskal–Wallis, and Chi-square for Independence tests as appropriate. Multivariate analysis using regression modeling was also used to identify predictors of outcomes.
RESULTS
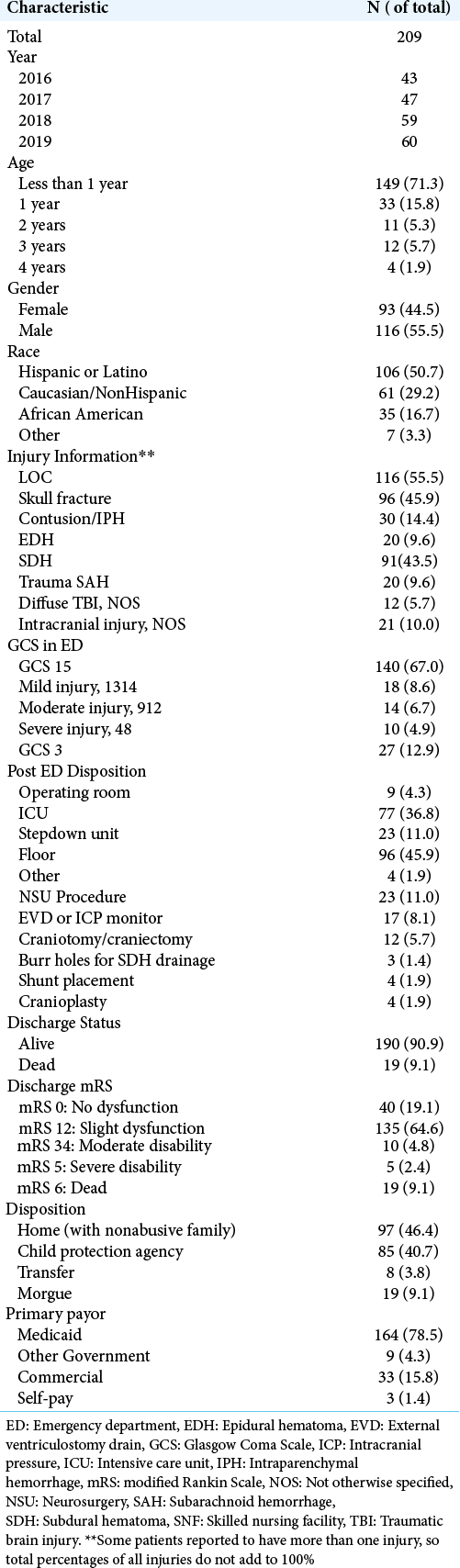
Our institution is the only level 1 pediatric trauma center in the region. Twenty percent of our patients are living in poverty, and 35% live in single parent households. There were 209 patients meeting inclusion criteria. The incidence of AHT is increasing over time (P < 0.001); [
Figure 4:
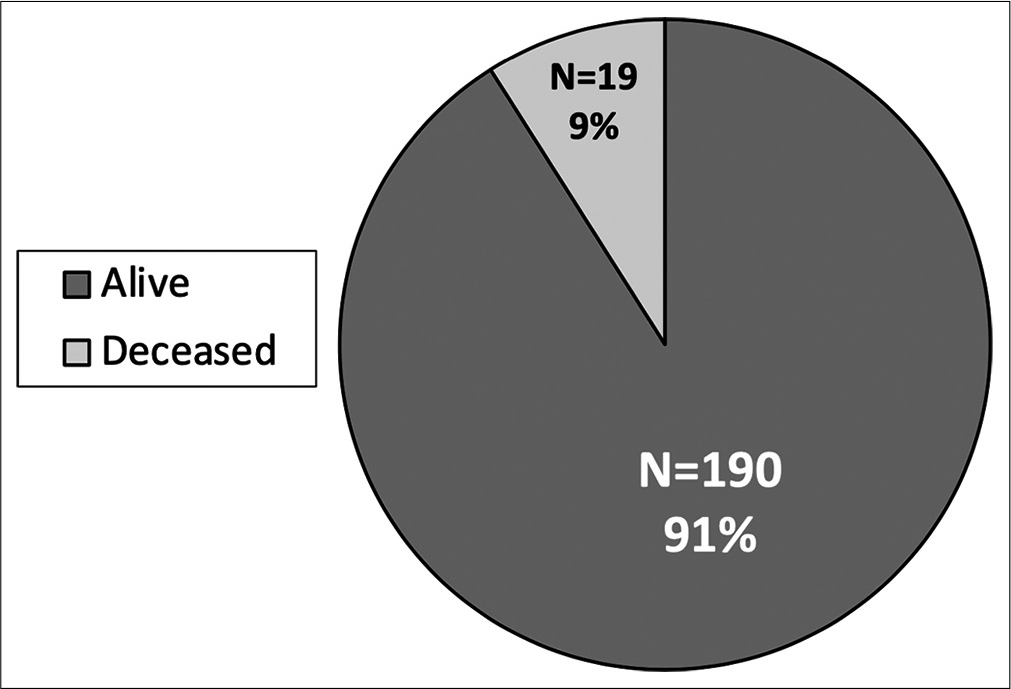
Rate of survival: high rate of mortality in pediatric abusive head trauma. There is a high rate of mortality in pediatric abusive head trauma with nearly 10% of abused children not surviving hospitalization. There are a total of 209 patients with 190 alive and 19 deceased children. All children who did not survive hospitalization presented as a Glasgow Coma Scale score of 3.
Figure 5:
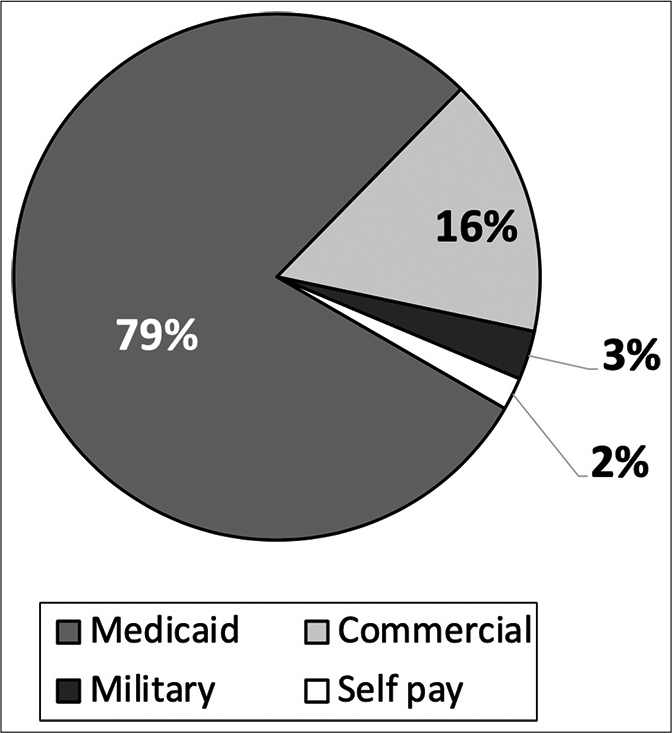
Primary payor information. Medicaid is the primary payor for nearly 80% of pediatric abusive head trauma at our institution. As hospital charges are not normally of $88,441 and an interquartile range of $43,247–226,937 per abused child per visit. This further supports the severity of many of these injuries.
Several injuries were associated with concurrent presence of other injuries. Patients with reported LOC had significantly higher rates of SDH (P < 0.001), contusions/intraparenchymal hemorrhage (IPH) (P = 0.001), EDH (P = 0.020), and traumatic SAH (P = 0.020). Patients with LOC were significantly less likely to have a skull fracture (P = 0.002). Patients with a skull fracture were significantly less likely to concurrently also have SDH (P < 0.001), diffuse traumatic brain injury (TBI) not otherwise specified (P = 0.007), and traumatic subarachnoid hemorrhage (SAH) (P = 0.014). The presence of SDH, EDH, or traumatic SAH was not associated with presence of another one of these injuries. Furthermore, reported LOC (P < 0.001), SDH (P < 0.001), traumatic SAH (P < 0.001), and diffuse TBI not otherwise specified (P < 0.001) were associated with the lower initial GCS. Skull fractures were associated with higher initial GCS (P < 0.001). There was no association between EDH (P = 0.366) and contusions/IPH (P = 0.351) with initial GCS.
In addition, several injuries were associated with increased likelihood of undergoing a neurosurgical intervention. Lower initial GCS was significantly associated with increased likelihood of undergoing any intervention (P < 0.001) as well as both EVD/ICP monitor (P < 0.001) and craniotomy/craniectomy (P < 0.001). In addition, there was a significant association between receiving EVD/ICP and craniotomy/craniectomy (P < 0.001). Patients with traumatic SAH (P < 0.001) and SDH (P = 0.002) were more likely to undergo an intervention. Although not reaching statistical significance, there was a trend towards increased interventions in patients with contusions/IPH (P = 0.089). EVD/ICP monitors were associated with the presence of traumatic SAH (P < 0.001) and SDH (P = 0.019). Although not statistically significant, there was a trend towards higher rates of EVD/ICP monitor placement in patients with contusions/IPH (P = 0.065). Craniotomy/craniectomy was more likely in patients with SDH (P = 0.002) and contusions/ IPH (P = 0.010). There was a trend toward increased craniotomy/craniectomy in patients with traumatic SAH, but this did not reach statistical significance. Patients with skull fractures were significantly less likely to undergo a neurosurgical intervention (P = 0.004), EVD/ICP monitor (P = 0.015), or craniotomy/craniectomy (P = 0.022). Interestingly, there was no association between EDH and undergoing an intervention (P = 0.548), EVD/ICP monitor (P = 0.590), or craniotomy/craniectomy (P = 0.812).
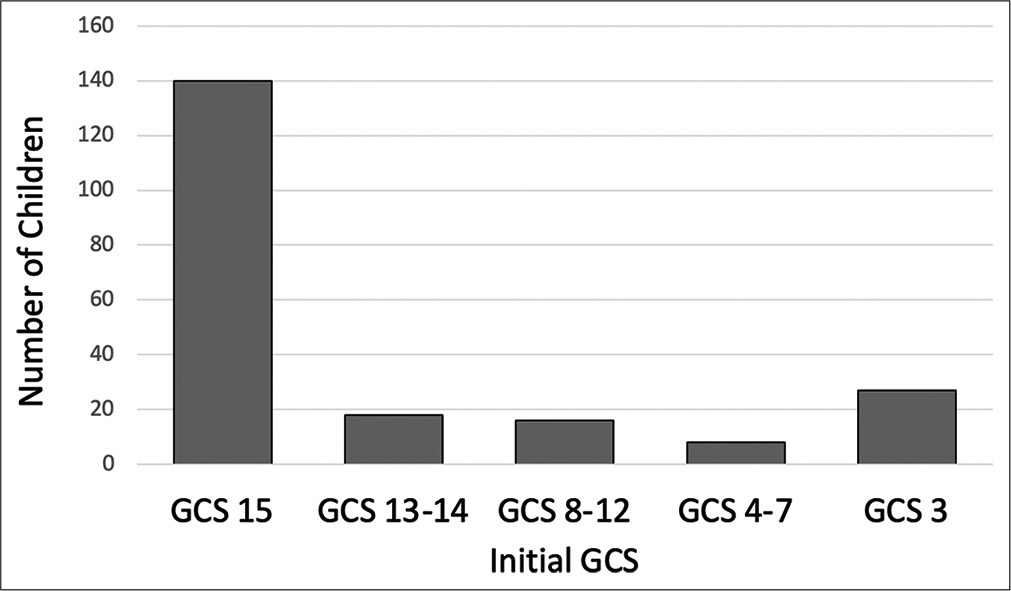
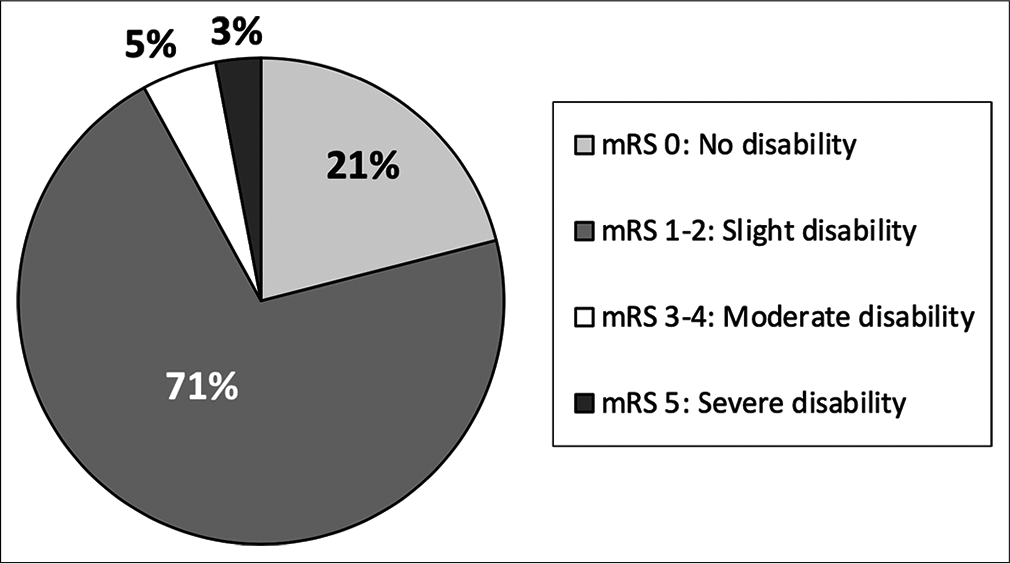
Nearly 10% of patients presenting with AHT did not survive. All of these patients presented with an initial GCS 3 and imaging findings more consistent with global diffuse anoxic brain injury. Increased mortality was associated with traumatic SAH (P < 0.001), and patients with skull fractures had decreased rates of mortality (P = 0.001). Higher mortality was seen in patients undergoing any neurosurgical intervention (P < 0.001), EVD/ICP monitor (P < 0.001), and craniotomy/craniectomy (P = 0.005). There is a high rate of morbidity and dysfunction in survivors of AHT with only 21% returning to neurologic baseline discharging with modified Rankin Score (mRS) of 0; however, the majority of patients were discharged with slight dysfunction, considered mRS 1–2 (67%) [
Multivariate analysis for predictors of outcome
Multivariate analysis was used to identify the most significant predictors of outcomes. Lower initial GCS (P < 0.001) and SDH (P = 0.017) are most predictive of undergoing a neurosurgical intervention. Similarly, lower initial GCS (P < 0.001) and SDH (P = 0.005) are most predictive of craniotomy/craniectomy, and lower initial GCS (P < 0.001) is the most predictive variable regarding EVD/ICP monitor placement. Initial GCS (P < 0.001), SDH (P = 0.020), and lack of a skull fracture (P = 0.045) are most predictive of lower functional outcome at discharge.
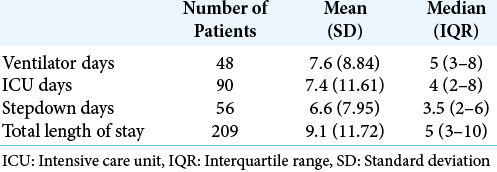
Multivariate analysis identified lower initial GCS (P < 0.001) and SDH (P = 0.001) as the largest predictors of longer ICU stay. Lower initial GCS (P < 0.001), diffuse TBI not otherwise specified (P = 0.003), and traumatic SAH (P = 0.029) are most predictive of longer days in stepdown. Variables most predictive of longer total length of stay are lower initial GCS (P < 0.001), diffuse TBI not otherwise specified (P = 0.003), SDH (P = 0.011), and lack of skull fracture (P < 0.020). Interestingly, length of stay in any unit was not associated with whether a neurosurgical intervention was performed or any specific intervention.
Multivariate analysis of hospital charges revealed that undergoing a neurosurgical intervention (P < 0.001), cranioplasty (P < 0.001), longer ICU stay (P < 0.001), longer total length of stay (P < 0.001), not surviving (P < 0.001), craniotomy/craniectomy (P = 0.017), and EVD/ICP monitor placement (P = 0.045) are most predictive of increased charges. Lower initial GCS or any specific injury was not predictive of hospital charges.
Subgroup analysis of AHT patients with radiographic evidence of intracranial hemorrhage or injury aside from skull fracture alone
There were 161 patients with AHT injuries aside from skull fracture alone. The age, gender, and racial characteristic compositions are largely unchanged regardless of including or excluding skull fracture alone. In this subgroup analysis, there are 34% of patients with skull fracture and concurrent intracranial hemorrhage or radiographic evidence of TBI. As expected, there are increased in the percentage of patients SDH (from 44% to 66%), contusion/IPH (14% to 22%), EDH (10% to 15%), and traumatic SAH (10% to 13%). Interestingly, there percentage of patients with LOC is increased in this subgroup despite excluding patients with LOC alone, suggesting that patients with LOC alone are likely to have sustained an intracranial injury as well.
DISCUSSION
The term AHT and the understanding of findings, circumstances, and biomechanics associated with AHT have evolved over time. In 1860, Auguste Ambroise Tardieu was the first to report the causal relationship between intracranial injury and physical abuse; however, his work was largely dismissed.[
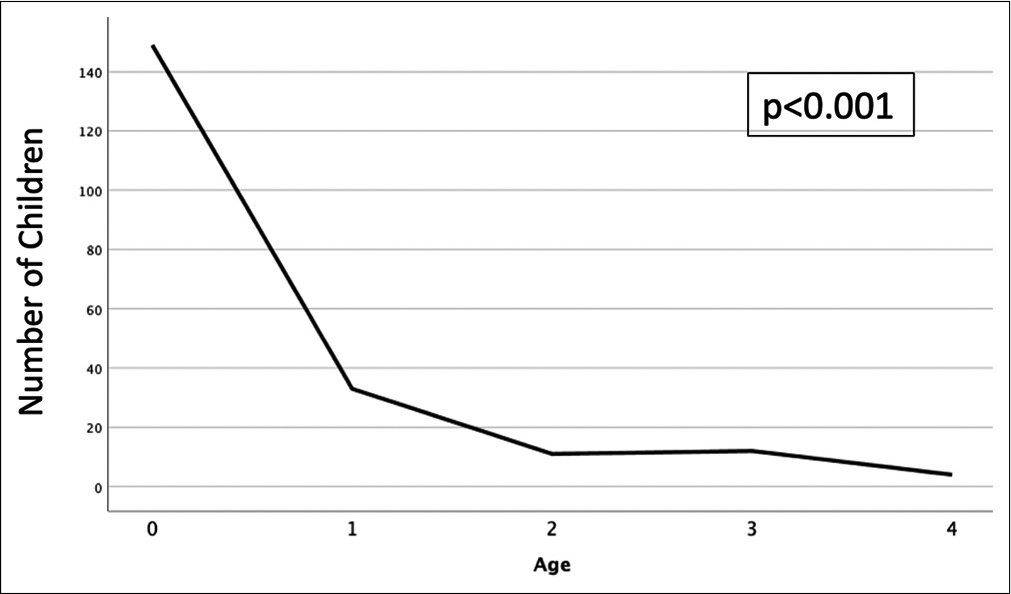
The incidence of AHT continues to increase over time with the majority of patients being <1 year of age.[
NAT can be challenging to diagnosis, especially AHT, with many children presenting with nonspecific symptoms, such as irritability or vomiting, and unreliable or absent history.[
Classically, SDH is well known consequence of trauma, and presence of a SDH in an infant is strongly associated with AHT and warrants a NAT evaluation.[
Multiple injuries are often present concurrently, which reiterates the need for meticulous examination to assess for additional bruising, external trauma, or other signs of injury in any patient suspected of AHT or NAT. Up to 85% of patients with SDH have retinal hemorrhages that can be identified with thorough examination.[
There are several known factors underlying the biomechanics of injury to the craniocervical junction in the pediatric population. Having a high head to body weight ratio, especially when the child’s head comprises 25% of its body weight, places the craniocervical junction, and therefore the high cervical cord, at a high risk for flexion and extensions injuries. The injury is further exacerbated when coupled with underdeveloped neck muscles and ligamentous laxity in this patient population.[
There are two phases of neurologic injury in victims of AHT. The primary injury is the immediate physical damage incurred by the abusive behavior itself. Following this trauma, secondary injury ensues as cerebral edema, hypoperfusion, hypoxia, ischemia, and oxidative stress develop and exacerbate neuronal damage and death.[
Often invasive neurosurgical procedures are performed as a life saving measure to prevent herniation and salvage uninjured brain in patients already severely injured from the primary traumatic event.[
Neurologic and functional outcomes following AHT are variable, relating to the severity of the initial injury.[
According to our data, length of stay is most predicted by the patient’s underlying injury, and accordingly, the neurologic status at initial presentation. Whether a patient underwent a neurosurgical procedure is not associated with length of stay, and this includes procedures that inherently imply a patient has a longer hospitalization, such as cranioplasty and shunt implantation. Hospital charges, however, are most predicted by length of stay as well as whether a patient had a neurosurgical intervention. Interestingly, not surviving was predictive of increased hospital charges. Although nonsurviving patients do not accrue costs through long hospital stay, these severely injured patients are most likely to undergo potentially lifesaving, also costly, interventions.
These findings reiterate that once the primary injury has occurred, neurosurgical intervention function to potentially decrease further damage from secondary injury, but they are not able to reverse the effects of the initial trauma. Despite many guidelines and management protocols relating appropriate triage and care of AHT patients once they arrive to the hospital, prevention is the most critical component of caring for these patients. Thus, it is imperative that neurosurgeons and other providers partner with the community, where the initial traumas occur, to educate and increase awareness about AHT.
Shaking and other abuse often results from desperate rage when the baby keeps crying and cannot be calmed or soothed. Infant crying is frequently reported as the inciting event of shaking, and interestingly, a study by Barr et al. found that the incidence of AHT by age closely mirrors the normal crying pattern curve for infants.[
Research has persistently found that socioeconomic status of the caretaker is a factor for AHT.[
Among other studies, a recent review by Lopes and de Albuquerque Williams investigated strategies to reduce NAT, including initiatives to reduce infant crying, regulate the caregiver’s emotional responses to crying, and raise awareness about pediatric AHT.[
Figure 7:
ThinkFirst: National injury prevention foundation. ThinkFirst is a nonprofit organization found over 30 years ago by the American Association of Neurological Surgeons and the Congress of Neurological Surgeons to partner with the community to raise awareness. ThinkFirst’s mission is to prevent brain, spinal cord, and other traumatic injuries through education, research, and advocacy.
ThinkFirst has a program tailored to educating and raising awareness about AHT, most specifically to prevent SBS. With this mission in mind, we have started ThinkFirst for Your Baby to provide a comprehensive review of infant safety-with a focus on prevention of AHT. At our institution, AHT is the leading cause of death among patients <2 years of age, and the incidence continue to rise. ThinkFirst aims to reduce the incidence of AHT by educating expectant parents on topics ranging from child development, stress coping mechanisms and most importantly, specific ways to comfort a crying baby while keeping oneself calm throughout the process. The senior author of this paper is the first physician in the United States trained to provide ThinkFirst for Your Baby seminars and partners with Loma Linda University Children’s Health to provide this free program within our community. A free course is offered to caregivers in the community to combat the exponential increase in AHT in the region. Courses provide detailed teaching and understanding for the care of infants, providing resources to caregivers, and emphasizing the disastrous consequences that are possible when a baby is shaken. By partnering with ThinkFirst, we can build on this existing framework and create new initiatives to further impact the community and prevent not only SHS but all AHT by educating and increasing public awareness through example. To reiterate, prevention of AHT is critical, and prevention is where neurosurgeons have the greatest opportunity to fight AHT. ThinkFirst is an exciting new initiative at our institution with evaluation of the efficacy of the program currently underway.
CONCLUSION
AHT has devastating neurologic consequences, and it continues to be a leading cause of death among infants and young children. Although a significant amount of research is being conducted in the area of pediatric TBI to elucidate its underlying the pathophysiology and discover novel treatment modalities for this patient population; prevention remains the best medicine. The population of patients with AHT is unique, and one that will benefit from continued multidisciplinary efforts to increase public awareness and educate parents and caregivers to prevent AHT.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982. 12: 557-63
2. Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet. 2000. 356: 1571-2
3. Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005. 116: e174-85
4. Barr RG, Trent RB, Cross J. Age-related incidence curve of hospitalized shaken baby syndrome cases: Convergent evidence for crying as a trigger to shaking. Child Abuse Negl. 2006. 30: 7-16
5. Bell E, Shouldice M, Levin AV. Abusive head trauma: A perpetrator confesses. Child Abuse Negl. 2011. 35: 74-7
6. Benzel EC, Hadden TA. Neurologic manifestations of child abuse. South Med J. 1989. 82: 1347-51
7. Berger RP, Fromkin JB, Stutz H, Makoroff K, Scribano PV, Feldman K. Abusive head trauma during a time of increased unemployment: A multicenter analysis. Pediatrics. 2011. 128: 637-43
8. Boop S, Axente M, Weatherford B, Klimo P. Abusive head trauma: An epidemiological and cost analysis. J Neurosurg Pediatr. 2016. 18: 542-9
9. Caffey J. The whiplash shaken infant syndrome: Manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics. 1974. 54: 396-403
10. Choudhary AK, Servaes S, Slovis TL, Palusci VJ, Hedlund GL, Narang SK. Consensus statement on abusive head trauma in infants and young children. Pediatr Radiol. 2018. 48: 1048-65
11. Christian CW, Block R. Committee on child abuse and neglect, American academy of pediatrics. Abusive head trauma in infants and children. Pediatrics. 2009. 123: 1409-11
12. Deans KJ, Thackeray J, Groner JI, Cooper JN, Minneci PC. Risk factors for recurrent injuries in victims of suspected non-accidental trauma: A retrospective cohort study. BMC Pediatr. 2014. 14: 217
13. Dornbos D, Gerhardstein D.editors. The Think First Foundation: Expanding the Footprint of Neurological Injury Prevention. 2019. p.
14. Duhaime AC, Christian CW. Abusive head trauma: Evidence, obfuscation, and informed management. J Neurosurg Pediatr. 2019. 24: 481-8
15. Ettaro L, Berger RP, Songer T. Abusive head trauma in young children: Characteristics and medical charges in a hospitalized population. Child Abuse Negl. 2004. 28: 1099-111
16. Fanconi M, Lips U. Shaken baby syndrome in Switzerland: Results of a prospective follow-up study, 2002-2007. Eur J Pediatr. 2010. 169: 1023-8
17. Feldman KW, Bethel R, Shugerman RP, Grossman DC, Grady MS, Ellenbogen RG. The cause of infant and toddler subdural hemorrhage: A prospective study. Pediatrics. 2001. 108: 636-46
18. Fitzpatrick MO, Maxwell WL, Graham DI. The role of the axolemma in the initiation of traumatically induced axonal injury. J Neurol Neurosurg Psychiatry. 1998. 64: 285-7
19. Gurdjian ES, Webster JE, Lissner HR. The mechanism of skull fracture. Radiology. 1950. 54: 313-39
20. Guthkelch AN. Subdural effusions in infancy: 24 cases. Br Med J. 1953. 1: 233-9
21. Handy TC, Nichols GR, Smock WS. Repeat visitors to a pediatric forensic medicine program. J Forensic Sci. 1996. 41: 841-4
22. Hettler J, Greenes DS. Can the initial history predict whether a child with a head injury has been abused?. Pediatrics. 2003. 111: 602-7
23. Hobbs C, Childs AM, Wynne J, Livingston J, Seal A. Subdural haematoma and effusion in infancy: An epidemiological study. Arch Dis Child. 2005. 90: 952-5
24. Ichord RN, Naim M, Pollock AN, Nance ML, Margulies SS, Christian CW. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: The role of diffusion-weighted imaging. J Neurotrauma. 2007. 24: 106-18
25. Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P. Subdural haemorrhages in infants: Population based study. BMJ. 1998. 317: 1558-61
26. Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay TC. Analysis of missed cases of abusive head trauma. JAMA. 1999. 281: 621-6
27. Johnson DL, Boal D, Baule R. Role of apnea in nonaccidental head injury. Pediatr Neurosurg. 1995. 23: 305-10
28. Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA. 2003. 290: 621-6
29. Kelly P, John S, Vincent AL, Reed P. Abusive head trauma and accidental head injury: A 20-year comparative study of referrals to a hospital child protection team. Arch Dis Child. 2015. 100: 1123-30
30. Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V. Neuroimaging: What neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011. 96: 1103-12
31. Kempe CH, Silverman FN, Steele BF, Droegemueller W, Silver HK. The battered-child syndrome. JAMA. 1962. 181: 17-24
32. Kesler H, Dias MS, Shaffer M, Rottmund C, Cappos K, Thomas NJ. Demographics of abusive head trauma in the commonwealth of Pennsylvania. J Neurosurg Pediatr. 2008. 1: 351-6
33. King WK, Kiesel EL, Simon HK. Child abuse fatalities: Are we missing opportunities for intervention?. Pediatr Emerg Care. 2006. 22: 211-4
34. Klevens J, Luo F, Xu L, Peterson C, Latzman NE. Paid family leave’s effect on hospital admissions for pediatric abusive head trauma. Inj Prev. 2016. 22: 442-5
35. Kriss VM, Kriss TC. SCIWORA (spinal cord injury without radiographic abnormality) in infants and children. Clin Pediatr (Phila). 1996. 35: 119-24
36. Labbé J. Ambroise Tardieu: the man and his work on child maltreatment a century before Kempe. Child Abuse Negl. 2005. 29: 311-24
37. Li F, Li H, Xiao Z, Lu R, Zhang Z, Zhu H. A review on injury mechanism of intracerebral hemorrhage in vehicle accidents. Curr Pharm Des. 2017. 23: 2177-92
38. Lopes NR, Williams LC. Pediatric abusive head trauma prevention initiatives: A literature review. Trauma Violence Abuse. 2018. 19: 555-66
39. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006. 147: S232-40
40. Maguire SA, Kemp AM, Lumb RC, Farewell DM. Estimating the probability of abusive head trauma: A pooled analysis. Pediatrics. 2011. 128: e550-64
41. Niederkrotenthaler T, Xu L, Parks SE, Sugerman DE. Descriptive factors of abusive head trauma in young children-United States, 2000-2009. Child Abuse Negl. 2013. 37: 446-55
42. Nuño M, Pelissier L, Varshneya K, Adamo MA, Drazin D. Outcomes and factors associated with infant abusive head trauma in the US. J Neurosurg Pediatr. 2015. 16: 515-22
43. Overpeck MD, Brenner RA, Trumble AC, Trifiletti LB, Berendes HW. Risk factors for infant homicide in the United States. N Engl J Med. 1998. 339: 1211-6
44. Parks S, Annest J, Hill H, Karch D.editors. Pediatric Abusive Head Trauma: Recommended Definitions for Public Health Surveillance and Research. 2012. p.
45. Pontarelli EM, Jensen AR, Komlofske KM, Bliss DW. Infant head injury in falls and nonaccidental trauma: Does injury pattern correlate with mechanism?. Pediatr Emerg Care. 2014. 30: 677-9
46. Reece RM, Sege R. Childhood head injuries: Accidental or inflicted?. Arch Pediatr Adolesc Med. 2000. 154: 11-5
47. Scheibl A, Calderón EM, Borau MJ, Prieto RM, González PF, Galiana GG. Epidural hematoma. J Pediatr Surg. 2012. 47: e19-21
48. Selassie AW, Borg K, Busch C, Russell WS. Abusive head trauma in young children: A population-based study. Pediatr Emerg Care. 2013. 29: 283-91
49. Sola R, Waddell VA, Peter SD, Aguayo P, Juang D. Non-accidental trauma: A national survey on management. Injury. 2018. 49: 921-6
50. Starling SP, Patel S, Burke BL, Sirotnak AP, Stronks S, Rosquist P. Analysis of perpetrator admissions to inflicted traumatic brain injury in children. Arch Pediatr Adolesc Med. 2004. 158: 454-8
51. Sun DTF, Zhu XL, Poon WS. Non-accidental subdural haemorrhage in Hong Kong: Incidence, clinical features, management and outcome. Childs Nerv Syst. 2006. 22: 593-8
52. Talvik I, Metsvaht T, Leito K, Põder H, Kool P, Väli M. Inflicted traumatic brain injury (ITBI) or shaken baby syndrome (SBS) in Estonia. Acta Paediatr. 2006. 95: 799-804
53. Thackeray J, Minneci PC, Cooper JN, Groner JI, Deans KJ. Predictors of increasing injury severity across suspected recurrent episodes of non-accidental trauma: A retrospective cohort study. BMC Pediatr. 2016. 16: 8
54. Theodore AD, Chang JJ, Runyan DK, Hunter WM, Bangdiwala SI, Agans R. Epidemiologic features of the physical and sexual maltreatment of children in the Carolinas. Pediatrics. 2005. 115: e331-7
55. Think First. Available from: http://www.thinkfirst.org [Last accessed on 2021 Feb 18].
56. Vinchon M, Defoort-Dhellemmes S, Desurmont M, Dhellemmes P. Accidental and nonaccidental head injuries in infants: A prospective study. J Neurosurg. 2005. 102: 380-4