- School of Medicine, St. George’s University, Great River, New York, United States,
- Department of Neurosurgery, SUNY Upstate Medical University, Syracuse, New York, United States.
Correspondence Address:
Disep I. Ojukwu, School of Medicine, St. George’s University, Great River, New York, United States.
DOI:10.25259/SNI_1254_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Disep I. Ojukwu1, Haydn A. Hoffman2, Rui Song2, Michael A. Galgano2. Intraspinal pilocytic astrocytomas: An overview and 2-D illustrative resection technique video. 11-Feb-2022;13:41
How to cite this URL: Disep I. Ojukwu1, Haydn A. Hoffman2, Rui Song2, Michael A. Galgano2. Intraspinal pilocytic astrocytomas: An overview and 2-D illustrative resection technique video. 11-Feb-2022;13:41. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11391
Abstract
Background: Spinal cord pilocytic astrocytomas (PAs) are rare and typically occur in pediatric patients. While PAs are often well-circumscribed and amenable to gross total resection, they sometimes harbor infiltrative components that can invade normal cord parenchyma.
Methods: Here, we present a 59-year-old female with a progressive right-sided hemi-sensory loss, right-sided hemiparesis, and gait imbalance. The preoperative T2 magnetic resonance imaging revealed a large loculated cystic tumor that focally compressed the dorsal medulla, while the contrast study revealed a 1.3 cm homogenously enhancing expansile intramedullary mass centered at the C1 level.
Results: The patient underwent a C1-2 laminectomy followed by gross total intramedullary tumor resection utilizing intraoperative dorsal column mapping. There were no operative complications. The patient had preserved motor strength and an expected dorsal column dysfunction, which largely resolved over 9 months postoperatively.
Conclusion: Here, we provide a broad overview of PAs, in addition to a case study/technical note that includes a 2-D intraoperative video detailing the resection technique.
Keywords: Craniovertebral junction tumor, Intradural intramedullary tumor, Neurosurgery spinal oncology, Pilocytic astrocytoma, Technical note surgical video
BACKGROUND AND IMPORTANCE
Pilocytic astrocytomas (PAs), which account for approximately 5% of all primary central nervous system tumors,[
Video 1
CLINICAL PRESENTATION
Patient presentation
A 59-year-old female presented with a several month history of progressive right-sided hemi-sensory loss, right-sided hemiparesis (4/5), and gait imbalance. The T2 magnetic resonance imaging (MRI) revealed a large cystic, loculated lesion that compressed the dorsal aspect of the medulla along with cord signal from C2 to C5 [
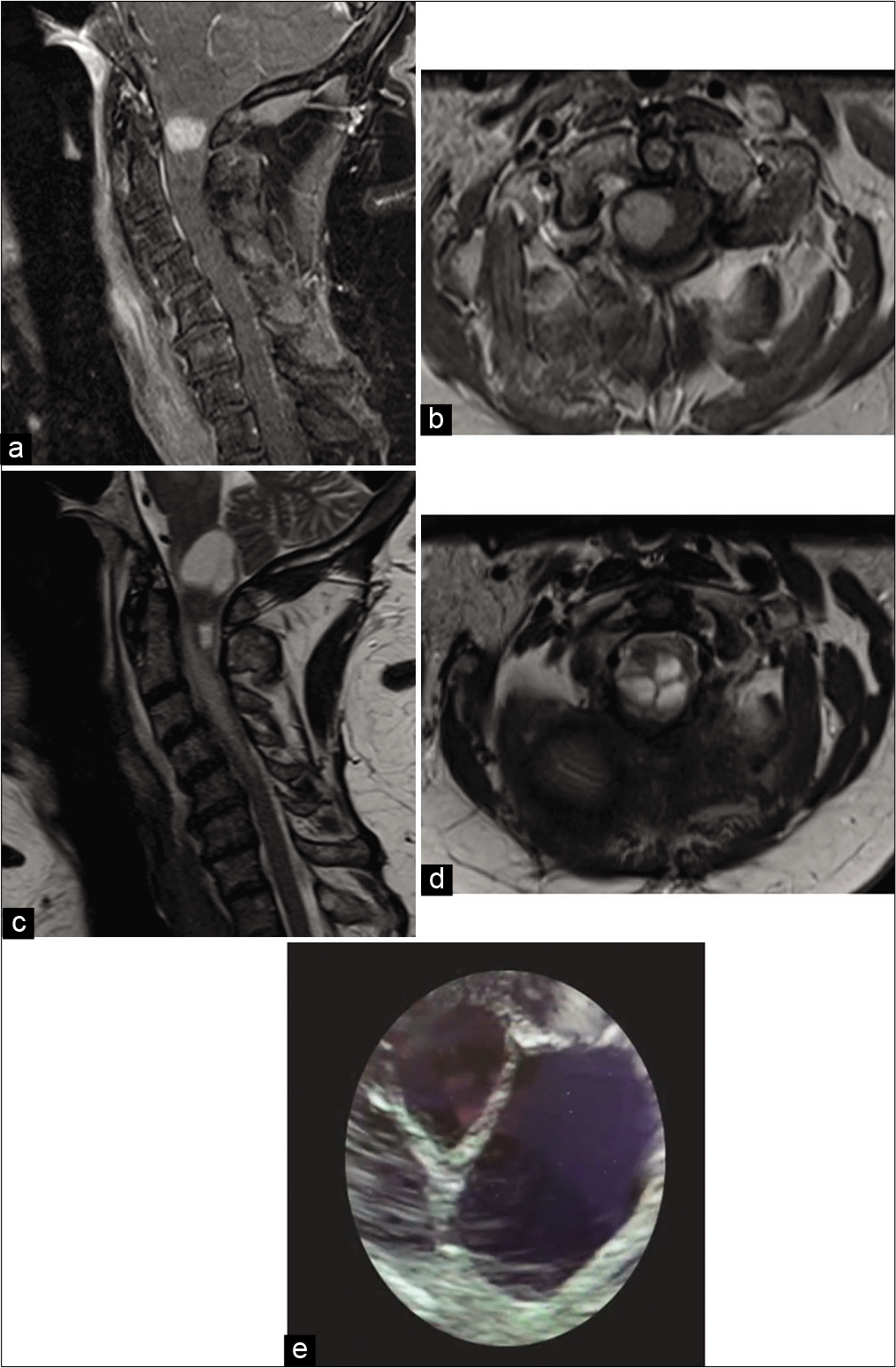
Figure 1:
Preoperative (a) sagittal T1 postcontrast magnetic resonance imaging (MRI), (b) axial T1 postcontrast MRI, (c) sagittal T2 MRI, and (d) axial T2 MRI demonstrating a 1.3 cm homogenously enhancing intramedullary mass centered at C1. Spinal cord T2 MRI signal change was present from C2 through C5. (e) Intraoperative ultrasound confirming cystic nature of C1 pilocytic astrocytoma (WHO Grade I).
Surgery
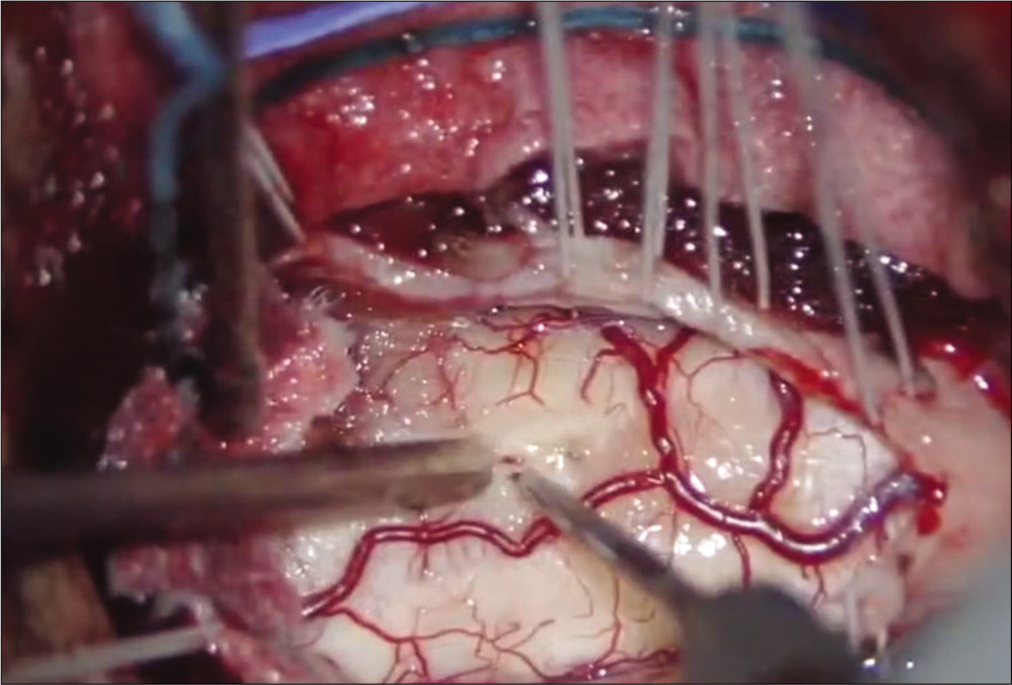
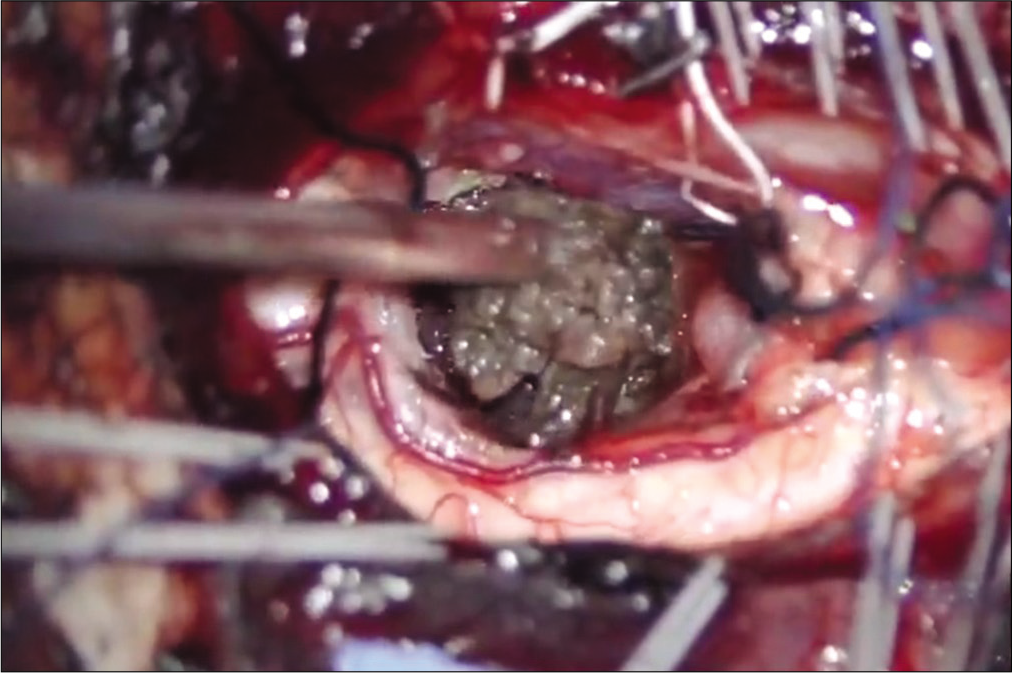
The patient was positioned prone on a “Jackson table” with intraoperative neurophysiologic monitoring – namely, intraoperative motor evoked potentials, somatosensory evoked potentials (SSEPs), free-running electromyography, and dorsal column mapping – utilized throughout the duration of surgery. The surgeon utilized a surgical microscope and ultrasound to perform a gross total resection (GTR) of the tumor. This required a C1-2 laminectomy, 3 cm suboccipital craniectomy, midline myelopathy (i.e., using the dorsal column mapping) [
Clinical outcome and pathology
Postoperatively, the patient had preserved motor strength and the expected dorsal column dysfunction, which markedly resolved within 9 months. The postoperative MRI scans demonstrated GTR of the tumor and dynamic X-rays confirmed no atlantoaxial or atlanto-occipital instability [
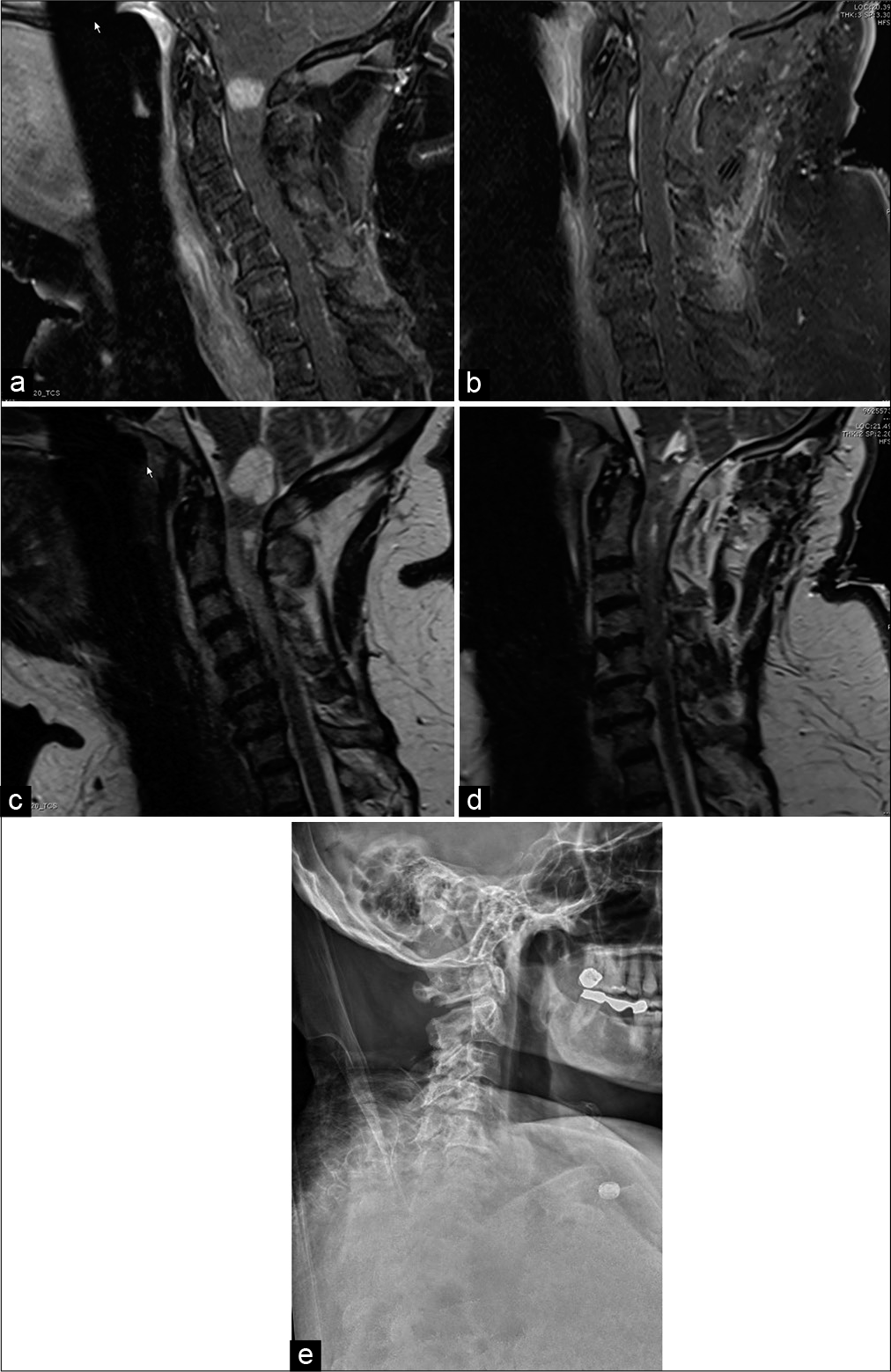
Figure 4:
Sagittal T1 postcontrast magnetic resonance imaging (MRI) (a) preoperative and (b) postoperative gross total resection of C1 pilocytic astrocytoma (WHO Grade I). Sagittal T2 MRI (c) preoperative and (d) postoperative resection of C1 pilocytic astrocytoma (WHO Grade I). Subtle postoperative hyperintensities within the tumor bed resulted from a combination of blood products and hemostatic agents. (e) Postoperative upright cervical spine X-ray (lateral view) showing no evidence of atlantoaxial or atlanto-occipital instability following gross total resection of C1 pilocytic astrocytoma (WHO Grade I).
DISCUSSION
Primary PA of the spine is extremely rare, particularly among adults, and GTR is the standard of care.[
CONCLUSION
In this technical note, we presented the surgical approach to resect an intramedullary occiput-C1-C2 PA.
Declaration of patient consent
Patient’s consent not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on:
References
1. Boschetti G, Santos AJ, Fermon KP, de Freitas Honório GL, Batistella G, Kusma SZ. Adult pilocytic astrocytomas: A Brazilian series. World Neurosurg. 2020. 133: e115-20
2. Jiang Y, Lv L, Yin S, Zhou P, Jiang S. Primary spinal pilocytic astrocytoma: Clinical study with long-term follow-up in 16 patients and a literature review. Neurosurg Rev. 2020. 43: 719-27
3. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: Analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol. 2012. 108: 187-93
4. Knight J, DeJesus O.editors. Pilocytic Astrocytoma. Treasure Island, FL: StatPearls Publishing; 2021. p.
5. Lee KJ, Marchan E, Peterson J, Harrell AC, Quinones-Hinojosa A, Brown PD. Management and survival of adult patients with pilocytic astrocytoma in the National Cancer Database. World Neurosurg. 2018. 112: e881-7
6. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-100
7. Stüer C, Vilz B, Majores M, Becker A, Schramm J, Simon M. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer. 2007. 110: 2799-808
8. Yanni DS, Ulkatan S, Deletis V, Barrenechea IJ, Sen C, Perin NI. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010. 12: 623-8