- Department of Neurosurgery, Jackson Memorial Hospital, University of Miami Miller School of Medicine, Miami, Florida, United States.
Correspondence Address:
Michael A. Silva, MD, Department of Neurosurgery, Jackson Memorial Hospital, University of Miami Miller School of Medicine, Miami, Florida, United States.
DOI:10.25259/SNI_1112_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael A. Silva, Stephanie Chen, Robert M. Starke. Unruptured cerebral aneurysm risk stratification: Background, current research, and future directions in aneurysm assessment. 29-Apr-2022;13:182
How to cite this URL: Michael A. Silva, Stephanie Chen, Robert M. Starke. Unruptured cerebral aneurysm risk stratification: Background, current research, and future directions in aneurysm assessment. 29-Apr-2022;13:182. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11562
Abstract
Background: The optimal management of unruptured cerebral aneurysms is widely debated in the medical field. Rapid technology advances, evolving understanding of underlying pathophysiology, and shifting practice patterns have made the cerebrovascular field particularly dynamic in recent years. Despite progress, there remains a dearth of large randomized studies to help guide the management of these controversial patients.
Methods: We review the existing literature on the natural history of unruptured cerebral aneurysms and highlight ongoing research aimed at improving our ability to stratify risk in these patients.
Results: Landmark natural history studies demonstrated the significance of size, location, and other risk factors for aneurysm rupture, but prior studies have significant limitations. We have begun to understand the underlying pathophysiology behind aneurysm formation and rupture and are now applying new tools such as flow dynamics simulations and machine learning to individualize rupture risk stratification.
Conclusion: Prior studies have identified several key risk factors for aneurysmal rupture, but have limitations. New technology and research methods have enabled us to better understanding individual rupture risk for patients with unruptured cerebral aneurysms.
Keywords: Aneurysm, Cerebrovascular, Computational flow dynamics, Endovascular, Machine learning, Open vascular neurosurgery, Subarachnoid hemorrhage
BACKGROUND
Cerebral aneurysms [
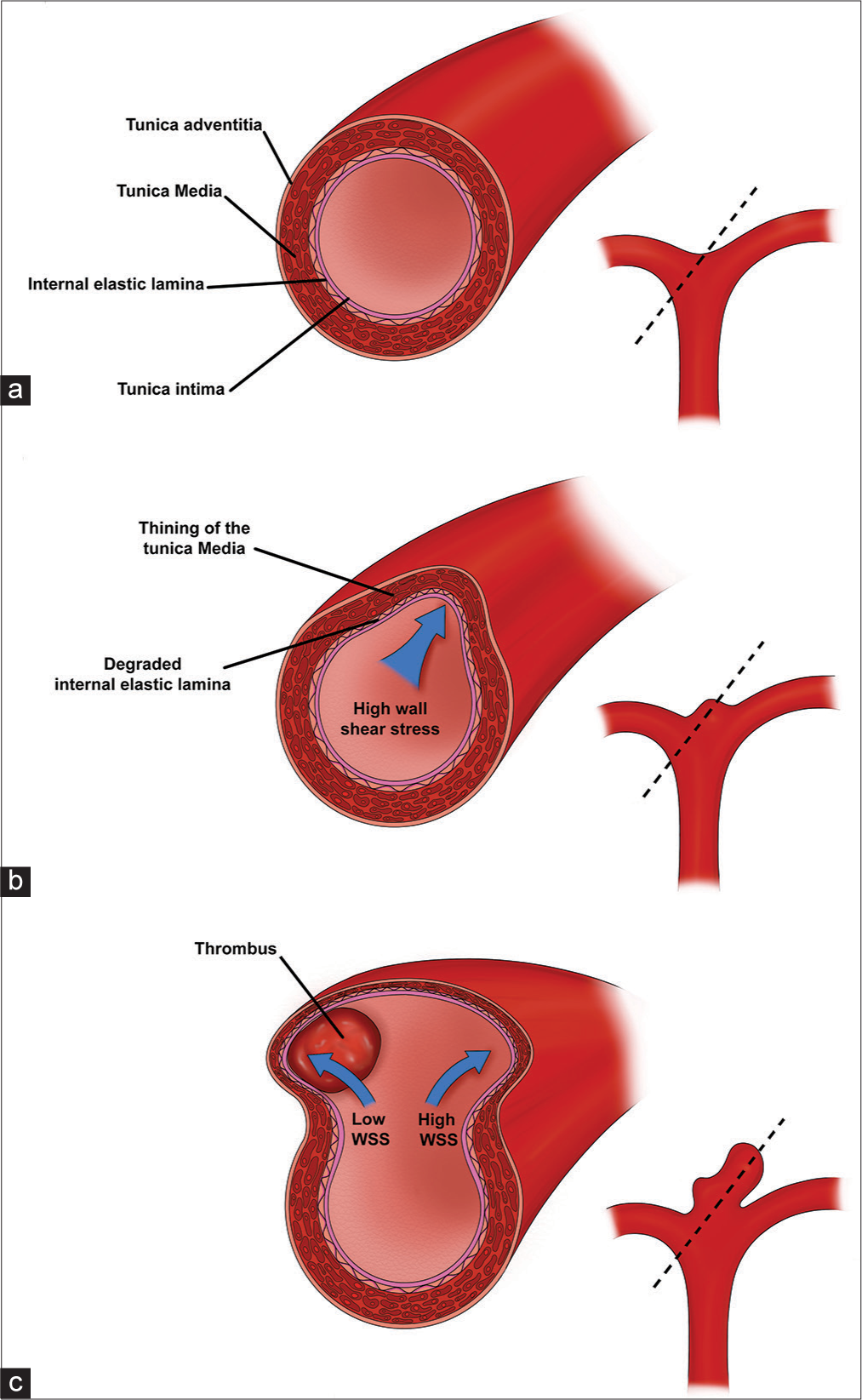
Figure 1:
Aneurysm wall pathophysiology and progression. (a) The normal arterial vessel wall consists of an inner endothelial layer (tunica intima) followed by the two strength layers: internal elastic lamina and the smooth-muscle of the tunica media. Dotted line represents the region of the cross-sectional image displayed on the left (b) Regions of high WSS (blue arrow) (e.g., vessel bifurcations and outer wall of curved vessels) trigger an inflammatory cascade, increased protease activity, and breakdown of the tunica media and internal elastic lamina. Loss of integrity of the strength layers of the vessel wall cause outpouching of the vessel wall and formation of an early aneurysm bulge. (c) Enlargement of the aneurysm sac alters the flow dynamics, leading to areas of low WSS and high WSS (blue arrows). Areas of low WSS can develop thrombus, which trigger further inflammation and wall breakdown. Areas of persistent high WSS continue to experience protease activity and inflammation leading to continues degradation of the tunica media and internal elastic lamina.
The rate of repeat hemorrhage after aSAH rises steadily for the first 7 days and then tapers at 3 weeks.[
NATURAL HISTORY OF UNRUPTURED INTRACRANIAL ANEURYSMS
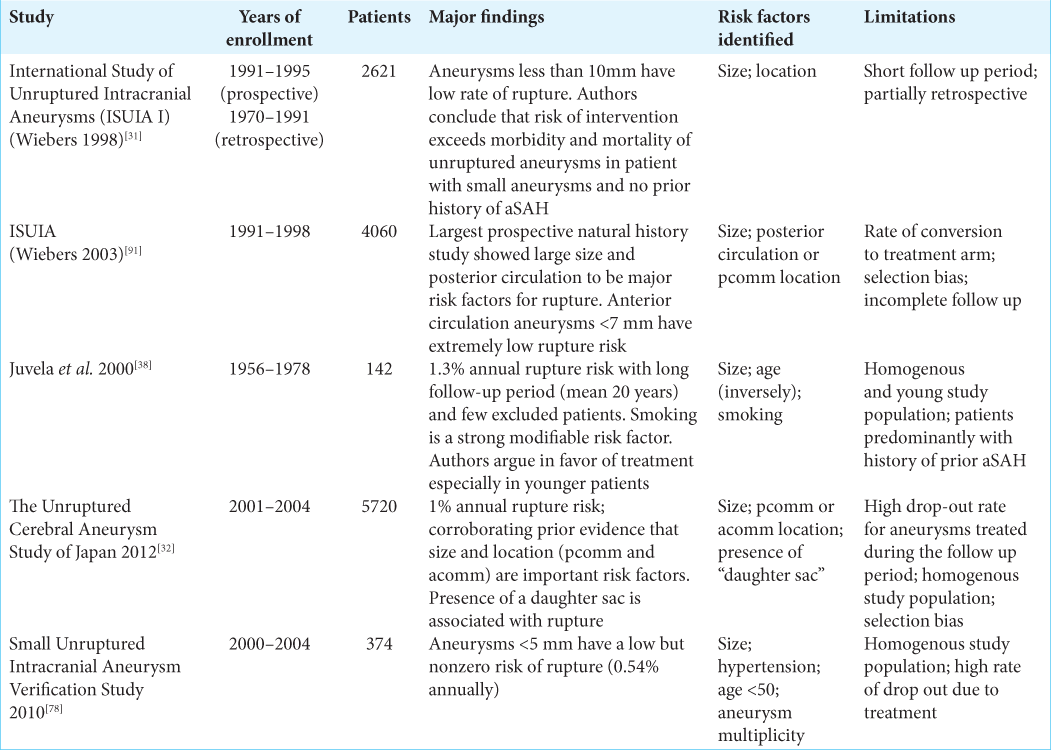
A series of landmark studies describe the natural history of unruptured cerebral aneurysms. The best-known of these studies is the International Study of Unruptured Intracranial Aneurysms (ISUIA) which was published in several phases throughout the late 1990s and early 2000s.
The ISUIA I study was a hybrid retrospective and prospective study published in 1998. In the retrospective portion, 1449 patients with 1937 unruptured cerebral aneurysms with a mean duration of follow-up of 8.3 years were analyzed. The study described a 0.05% annual risk of rupture for aneurysms <10 mm (727 patients), which rose to 0.5% among patients with a prior history of ruptured aneurysm (722 patients). Rupture risk increased with aneurysm diameter, with aneurysms larger than 25 mm carrying a 6% annual risk of rupture.[
The ISUIA 2 study assessed 4060 patients (1692 managed conservatively, 1917 with open surgery, 451 endovascular treatment) with unruptured aneurysms. Importantly, ISUIA investigated the impact of both size and location on 5-year cumulative rupture risk. The results corroborated earlier findings that larger size was associated with increased rupture risk, concluding that anterior circulation aneurysms <7 mm had a 0% rupture risk over 5 years. In contrast, posterior circulation and posterior communicating artery aneurysms of at least 25 mm had a 50% 5-year cumulative rupture rate.[
Natural history studies out of Finland (Juvela et al., 2000) and Japan (Unruptured Cerebral Aneurysm Study of Japan [UCAS], 2012) reported similar findings with additional nuances. Juvela et al. followed 2575 patients over nearly 20 years.[
The UCAS study was a prospective natural history study from Japan.[
To consolidate these findings Greving et al. performed a meta-analysis from 6 prospective natural history studies[
A summary of the major natural history studies of unruptured cerebral aneurysms is shown in [
ANEURYSM SIZE
The debate over the importance of aneurysm size in predicting rupture risk is ongoing. The natural history studies reviewed above consistently show that increased aneurysm size is a risk factor for rupture. ISUIA, which reported a 0% rate of rupture for aneurysms <7 mm in the anterior circulation,[
Reconciling this data is the subject of ongoing debate. It has been proposed that post-rupture aneurysm size may not represent the size of an aneurysm at the time of rupture, suggesting that perhaps the small ruptured aneurysms we see were actually larger at the time of rupture. However, Rahman et al. evaluated imaging of 13 patients who had pre- and post-rupture imaging and found that none had significantly decreased in size and in fact 53.8% increased in size after rupture.[
The constancy of aneurysm growth has also been called to question. Aneurysm growth has been shown to be associated with rupture.[
Moreover, it is impossible to know the true denominator when estimating the risk of rupture of small aneurysms. Autopsy studies, radiographic surveys, and meta-analyses have consistently shown a high percentage of small aneurysms. Dr. Housepian’s autopsy data from the 1910s to 1950s found that 45% of the aneurysms found among his nearly 6000 brain autopsies were <6 mm.[
The Small Unruptured Intracranial Aneurysm Verification Study out of Japan in 2010 sought to address this very issue. This prospective study of 374 patients with aneurysms <5 mm found a 0.5% overall annual rupture risk, further demonstrating the nonzero risk of rupture for small aneurysms.[
In summary, the literature on small aneurysms suggests that they may have a very low rupture risk. However, given the high morbidity and mortality associated with rupture in combination with the high prevalence of small aneurysms (likely over 50%), further studies are necessary to weigh the risks and benefits of treatment versus observation.
HERITABILITY AND FAMILY HISTORY
As many as, 10–20% of patients with aSAH have a positive family history of aneurysmal rupture.[
MODIFIABLE RISK FACTORS
Hypertension and smoking have been demonstrated by several studies to be associated with increased risk of aneurysmal rupture.[
LOCATION
The major natural history studies vary in their classification of aneurysm location, thus their cumulative results leave room for interpretation. ISUIA found that pcomm and posterior circulation aneurysms (as a group) had a higher rate of rupture than anterior circulation (which included acomm, MCA, and noncavernous ICA aneurysms).[
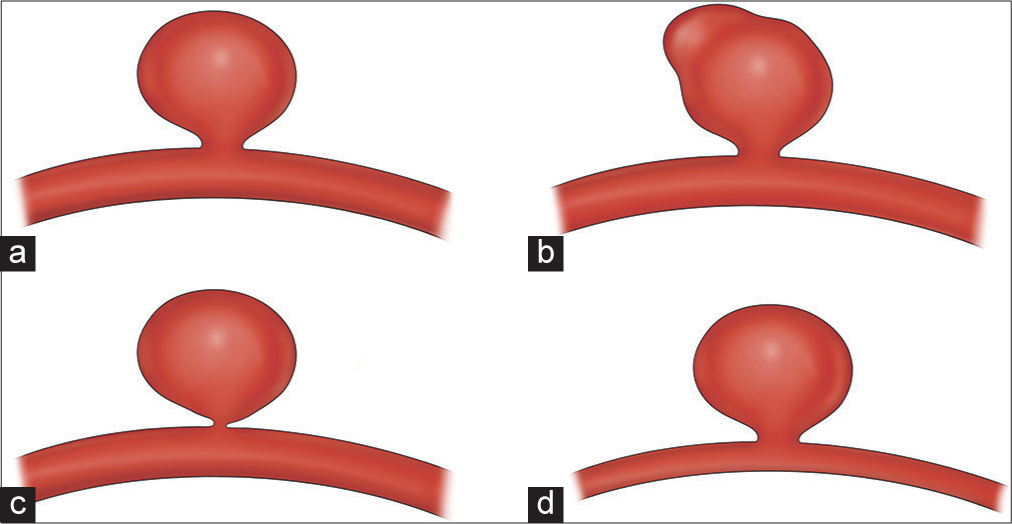
MORPHOLOGY
The only major natural history study to find a significant association between a morphologic feature and aneurysm rupture risk is UCAS, which found that specifically the presence of a daughter sac in the aneurysm was high risk [
Certain morphologies have been associated with aneurysm growth, which we know is associated with aneurysmal rupture.[
A recent study from China found that aneurysm sac irregularity (lobulation or blebs) and higher aspect ratio was associated with rupture.[
It has also been proposed that aneurysm size and morphology may be linked in that large aneurysms are more likely to develop blebs and other high-risk morphologies compared to small aneurysms, which become more regular with growth.[
FLOW DYNAMICS
Research has increasingly focused on flow dynamics as a possible explanation for why some aneurysms are more likely to rupture than others. Several parameters have been highlighted in the literature as important. In a matched case– control study, Skodvin et al. identified a straighter inflow angle as significantly associated with ruptured cases.[
Higher aspect ratio [i.e., the ratio of aneurysm dome diameter to neck width,
Higher size ratio [i.e., the ratio of the maximum diameter of the aneurysm sac to the diameter of the parent vessel,
WSS is an important concept that is now being applied to aneurysm modeling to better understand rupture risk. WSS represents the frictional force on the wall per unit area and is dependent on blood viscosity and velocity.[
A similar concept called oscillatory shear index (OSI) measures the change in the vector of blood flow throughout the cardiac cycle, a more physiologic measure of WSS in the vasculature.[
ANEURYSM PATHOGENESIS
Cerebral arteries consist of several layers that are critical for understanding the pathophysiology of aneurysm formation. The innermost layer, the tunica intima, consists of endothelium, and basement membrane. The tunica intima is surrounded by an internal elastic lamina, which serves as a strength layer to withstand the repeated stretch forces from each cardiac cycle. This is followed by a thick tunica media of smooth muscle and then the thin adventitia (which is present in the proximal cerebral vessels but not distally); there is no external elastic lamina [
In vivo animal models and human histologic studies have facilitated our understanding of this process. Meng et al. used an in vivo dog model to surgically create artificial vessel bifurcations and subsequently measured hemodynamics, WSS, and histologic changes over time.[
Once an early aneurysm sac has appeared, flow dynamics change dramatically [
RADIOGRAPHIC PREDICTION
MRI is increasingly being used to detect aneurysms at higher risk of rupture. It has been shown that gadolinium enhancement of the aneurysm wall on MRI is associated with areas of low WSS.[
More advanced imaging modalities have shown promise in identifying unstable aneurysms and predicting future rupture. Given the known role of inflammation in the development of the aneurysm sac,[
Ferumoxytol is an FDA-approved iron oxide used for iron deficiency anemia, which appears hyperintense on T1 and hypointense on T2 gradient echo sequences. It is cleared by macrophages and is, therefore, useful for identifying regions of active inflammation.[
MACHINE LEARNING (ML)
Due to the complexity of risk stratification for aSAH, some groups have turned to novel data analytics tools to better understand aneurysm rupture risk. ML algorithms, of which there are many types, have revolutionized high throughput data analysis because of their ability to detect patterns in data that traditional statistics may not.[
ML is increasingly used throughout medicine, neurosurgery, and has even integrated itself into many aspects of our daily life.[
CONCLUSION
Our understanding of unruptured cerebral aneurysms and their risk of rupture has improved over the past several decades. However, it remains challenging to identify aneurysms at high risk for rupture and to individualize risk stratification for unruptured aneurysms. Natural history data tells us that size, location, and family history, among other variables, are important for estimating rupture risk. Newer tools such as flow dynamics analysis, in vivo aneurysm models, and ML have improved our ability to predict which aneurysms are at higher risk of rupture. Despite significant progress, there remains uncertainty and debate regarding the natural history of unruptured cerebral aneurysms, even among cerebrovascular specialists.[
As the cerebrovascular field continues to evolve, we will continue to see the expansion of endovascular treatment options[
Disclosures
RMS research is supported by the NREF, Joe Niekro Foundation, Brain Aneurysm Foundation, Bee Foundation, and by National Institute of Health (R01NS111119-01A1) and (UL1TR002736, KL2TR002737) through the Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. RMS has an unrestricted research grant from Medtronic and has consulting and teaching agreements with Penumbra, Abbott, Medtronic, InNeuroCo and Cerenovus.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal N, Gala NB, Choudhry OJ, Assina R, Prestigiacomo CJ, Duffis EJ. Prevalence of asymptomatic incidental aneurysms: A review of 2,685 computed tomographic angiograms. World Neurosurg. 2014. 82: 1086-90
2. Alvord EC, Loeser JD, Bailey WL, Copass MK. Subarachnoid hemorrhage due to ruptured aneurysms. A simple method of estimating prognosis. Arch Neurol. 1972. 27: 273-84
3. Backes D, Rinkel GJ, Greving JP, Velthuis BK, Murayama Y, Takao H. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 2017. 88: 1600-6
4. Bijlenga P, Gondar R, Schilling S, Morel S, Hirsch S, Cuony J. PHASES score for the management of intracranial aneurysm: A cross-sectional population-based retrospective study. Stroke. 2017. 48: 2105-12
5. Blankena R, Kleinloog R, Verweij BH, van Ooij P, Ten Haken B, Luijten PR. Thinner regions of intracranial aneurysm wall correlate with regions of higher wall shear stress: A 7T MRI study. AJNR Am J Neuroradiol. 2016. 37: 1310-7
6. Bond KM, Krings T, Lanzino G, Brinjikji W. Intracranial dissections: A pictorial review of pathophysiology, imaging features, and natural history. J Neuroradiol. 2021. 48: 176-88
7. Brinjikji W, Pereira VM, Khumtong R, Kostensky A, Tymianski M, Krings T. PHASES and ELAPSS scores are associated with aneurysm growth: A study of 431 unruptured intracranial aneurysms. World Neurosurg. 2018. 114: e425-32
8. Broderick JP, Brown RD, Sauerbeck L, Hornung R, Huston J, Woo D. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009. 40: 1952-7
9. Brown RD, Huston J, Hornung R, Foroud T, Kallmes DF, Kleindorfer D. Screening for brain aneurysm in the Familial Intracranial Aneurysm study: Frequency and predictors of lesion detection. J Neurosurg. 2008. 108: 1132-8
10. Burns JD, Huston J, Layton KF, Piepgras DG, Brown RD. Intracranial aneurysm enlargement on serial magnetic resonance angiography: Frequency and risk factors. Stroke. 2009. 40: 406-11
11. Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg. 2015. 61: 1068-80
12. Chalouhi N, Jabbour P, Magnotta V, Hasan D. The emerging role of ferumoxytol-enhanced MRI in the management of cerebrovascular lesions. Molecules. 2013. 18: 9670-83
13. Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999. 45: 1137-46
14. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/ american Stroke Association. Stroke. 2012. 43: 1711-37
15. Dell S. Asymptomatic cerebral aneurysm: Assessment of its risk of rupture. Neurosurgery. 1982. 10: 162-6
16. Dumont AS, Lanzino G, Kassell NF. Unruptured aneurysms. J Neurosurg. 2002. 96: 52-6
17. Etminan N, Beseoglu K, Barrow DL, Bederson J, Brown RD, Connolly ES. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: Proposal of an international research group. Stroke. 2014. 45: 1523-30
18. Etminan N, Beseoglu K, Steiger HJ, Hanggi D. The impact of hypertension and nicotine on the size of ruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2011. 82: 4-7
19. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005. 36: 2773-80
20. Fennell VS, Martirosyan NL, Palejwala SK, Lemole GM, Dumont TM. Morbidity and mortality of patients with endovascularly treated intracerebral aneurysms: Does physician specialty matter?. J Neurosurg. 2016. 124: 13-7
21. Foreman PM, Hendrix P, Harrigan MR, Fisher WS, Vyas NA, Lipsky RH. PHASES score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. 2018. 53: 69-73
22. Frosen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019. 47: E21
23. Gieteling EW, Rinkel GJ. Characteristics of intracranial aneurysms and subarachnoid haemorrhage in patients with polycystic kidney disease. J Neurol. 2003. 250: 418-23
24. Graf CJ. Prognosis for patients with nonsurgically-treated aneurysms. Analysis of the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. J Neurosurg. 1971. 35: 438-43
25. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
26. Hainc N, Mannil M, Anagnostakou V, Alkadhi H, Bluthgen C, Wacht L. Deep learning based detection of intracranial aneurysms on digital subtraction angiography: A feasibility study. Neuroradiol J. 2020. 33: 311-7
27. Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: A pilot study. Stroke. 2012. 43: 3258-65
28. Hijdra A, Braakman R, van Gijn J, Vermeulen M, van Crevel H. Aneurysmal subarachnoid hemorrhage. Complications and outcome in a hospital population. Stroke. 1987. 18: 1061-7
29. Hitchcock E, Gibson WT. A review of the genetics of intracranial berry aneurysms and implications for genetic counseling. J Genet Couns. 2017. 26: 21-31
30. Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958. 17: 409-23
31. 32. Investigators UJ, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82 33. Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H. Unruptured intracranial aneurysms: Incidence of rupture and risk factors. Stroke. 2009. 40: 313-6 34. Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among medicare beneficiaries, 2000 to 2010. Stroke. 2015. 46: 2452-7 35. Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998. 50: 1413-8 36. Jordan MI, Mitchell TM. Machine learning: Trends, perspectives, and prospects. Science. 2015. 349: 255-60 37. Juvela S, Korja M. Intracranial aneurysm parameters for predicting a future subarachnoid hemorrhage: A long-term follow-up study. Neurosurgery. 2017. 81: 432-40 38. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J Neurosurg. 2000. 93: 379-87 39. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J Neurosurg. 2008. 108: 1052-60 40. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke. 2013. 44: 2414-21 41. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001. 32: 485-91 42. Juvela S. Growth and rupture of unruptured intracranial aneurysms. J Neurosurg. 2018. 131: 843-51 43. Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000. 31: 392-7 44. Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: Exploring constancy. J Neurosurg. 2008. 109: 176-85 45. Korja M, Kivisaari R, Jahromi BR, Lehto H. Size and location of ruptured intracranial aneurysms: Consecutive series of 1993 hospital-admitted patients. J Neurosurg. 2017. 127: 748-53 46. Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke. 2014. 45: 1958-63 47. Lanzino G, Brown RD. Natural history of unruptured intracranial aneurysms. J Neurosurg. 2012. 117: 50-1 48. Leemans EL, Cornelissen BM, Said M, van den Berg R, Slump CH, Marquering HA. Intracranial aneurysm growth: Consistency of morphological changes. Neurosurg Focus. 2019. 47: E5 49. Luther E, McCarthy DJ, Brunet MC, Sur S, Chen SH, Sheinberg D. Treatment and diagnosis of cerebral aneurysms in the post-international subarachnoid aneurysm trial (ISAT) era: Trends and outcomes. J Neurointerv Surg. 2020. 12: 682-7 50. Majidi SM, Mehta A, De Leacy R, Mocco J, Fifi J. Trend in mortality and morbidity after treatment of unruptured intracranial aneurysm in the United States 2006-2016. J Neurointerv Surg. 2020. p. 51. Maleki F, Ovens K, Najafian K, Forghani B, Reinhold C, Forghani R. Overview of machine learning part 1: Fundamentals and classic approaches. Neuroimaging Clin N Am. 2020. 30: e17-32 52. McDowell MM, Zhao Y, Kellner CP, Barton SM, Sussman E, Claassen J. Demographic and clinical predictors of multiple intracranial aneurysms in patients with subarachnoid hemorrhage. J Neurosurg. 2018. 128: 961-8 53. Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014. 35: 1254-62 54. Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007. 38: 1924-31 55. Mitchell P, Jakubowski J. Estimate of the maximum time interval between formation of cerebral aneurysm and rupture. J Neurol Neurosurg Psychiatry. 2000. 69: 760-7 56. Nakagawa D, Cushing C, Nagahama Y, Allan L, Hasan D. Quantitative susceptibility mapping as a possible tool to radiographically diagnose sentinel headache associated with intracranial aneurysm: Case report. World Neurosurg. 2017. 103: 954.e1-4 57. Noble WS. What is a support vector machine?. Nat Biotechnol. 2006. 24: 1565-7 58. Perera R, Isoda H, Ishiguro K, Mizuno T, Takehara Y, Terada M. Assessing the risk of intracranial aneurysm rupture using morphological and hemodynamic biomarkers evaluated from magnetic resonance fluid dynamics and computational fluid dynamics. Magn Reson Med Sci. 2020. 19: 333-44 59. Phillips LH, Whisnant JP, O’Fallon WM, Sundt TM. The unchanging pattern of subarachnoid hemorrhage in a community. Neurology. 1980. 30: 1034-40 60. Post KD, Flamm ES, Goodgold A, Ransohoff J. Ruptured intracranial aneurysms. Case morbidity and mortality. J Neurosurg. 1977. 46: 290-5 61. Rahman M, Ogilvy CS, Zipfel GJ, Derdeyn CP, Siddiqui AH, Bulsara KR. Unruptured cerebral aneurysms do not shrink when they rupture: Multicenter collaborative aneurysm study group. Neurosurgery. 2011. 68: 155-60 62. Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A. Size ratio correlates with intracranial aneurysm rupture status: A prospective study. Stroke. 2010. 41: 916-20 63. Raymond J, Guillemin F, Proust F, Molyneux AJ, Fox AJ, Claiborne JS. A critical review of the international study of unruptured intracranial aneurysms (ISUIA) and of appropriate methods to address the clinical problem. Interv Neuroradiol. 2008. 14: 85-96 64. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke. 1998. 29: 251-6 65. Roethlisberger M, Achermann R, Bawarjan S, Stienen MN, Fung C, D’Alonzo D. Predictors of occurrence and anatomic distribution of multiple aneurysms in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018. 111: e199-205 66. Rutledge C, Jonzzon S, Winkler EA, Raper D, Lawton MT, Abla AA. Small aneurysms with low PHASES scores account for most subarachnoid hemorrhage cases. World Neurosurg. 2020. 139: e580-4 67. Salahuddin H, Siddiqui NS, Castonguay AC, Johnson M, Zaidi SF, Jumaa MA. Recent trends in electively treated unruptured intracranial aneurysms. J Stroke Cerebrovasc Dis. 2019. 28: 2011-7 68. Sandvei MS, Romundstad PR, Muller TB, Vatten L, Vik A. Risk factors for aneurysmal subarachnoid hemorrhage in a prospective population study: The HUNT study in Norway. Stroke. 2009. 40: 1958-62 69. Sato K, Yoshimoto Y. Risk profile of intracranial aneurysms: Rupture rate is not constant after formation. Stroke. 2011. 42: 3376-81 70. Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: A community-based study. J Neurosurg. 1995. 83: 426-9 71. Senders JT, Staples PC, Karhade AV, Zaki MM, Gormley WB, Broekman ML. Machine learning and neurosurgical outcome prediction: A systematic review. World Neurosurg. 2018. 109: 476-86.e1 72. Seule M, Oswald D, Muroi C, Brandi G, Keller E. Outcome, return to work and health-related costs after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2020. 33: 49-57 73. Sheinberg DL, McCarthy DJ, Elwardany O, Bryant JP, Luther E, Chen SH. Endothelial dysfunction in cerebral aneurysms. Neurosurg Focus. 2019. 47: E3 74. Shi Z, Chen GZ, Mao L, Li XL, Zhou CS, Xia S. Machine learning-based prediction of small intracranial aneurysm rupture status using CTA-derived hemodynamics: A multicenter study. AJNR Am J Neuroradiol. 2021. 42: 648-54 75. Shishir SS, Karim A, Islam AK, Hasan AB. Blood flow dynamics in cerebral aneurysm. Proc Eng. 2015. 105: 919-27 76. Silva MA, Patel J, Kavouridis V, Gallerani T, Beers A, Chang K. Machine learning models can detect aneurysm rupture and identify clinical features associated with rupture. World Neurosurg. 2019. 131: e46-51 77. Skodvin TO, Evju O, Sorteberg A, Isaksen JG. Prerupture intracranial aneurysm morphology in predicting risk of rupture: A matched case-control study. Neurosurgery. 2019. 84: 132-40 78. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010. 41: 1969-77 79. Starke RM, Komotar RJ, Otten ML, Schmidt JM, Fernandez LD, Rincon F. Predicting long-term outcome in poor grade aneurysmal subarachnoid haemorrhage patients utilising the glasgow coma scale. J Clin Neurosci. 2009. 16: 26-31 80. Starke RM. Small cerebral aneurysms: Can we identify patients at risk for rupture?. Ann Intern Med. 2017. 167: 59-60 81. Sundt TM, Whisnant JP. Subarachnoid hemorrhage from intracranial aneurysms. Surgical management and natural history of disease. N Engl J Med. 1978. 299: 116-22 82. Teunissen LL, Rinkel GJ, Algra A, van Gijn J. Risk factors for subarachnoid hemorrhage: A systematic review. Stroke. 1996. 27: 544-9 83. Tremmel M, Dhar S, Levy EI, Mocco J, Meng H. Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: Results from a virtual experimental study. Neurosurgery. 2009. 64: 622-30 84. Turan N, Heider RA, Roy AK, Miller BA, Mullins ME, Barrow DL. Current perspectives in imaging modalities for the assessment of unruptured intracranial aneurysms: A comparative analysis and review. World Neurosurg. 2018. 113: 280-92 85. Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm?. Neurosurgery. 2001. 48: 495-502 86. Vergouwen MD, Backes D, van der Schaaf IC, Hendrikse J, Kleinloog R, Algra A. Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: A follow-up study. AJNR Am J Neuroradiol. 2019. 40: 1112-6 87. Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: Growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013. 269: 258-65 88. Virta JJ, Satopaa J, Luostarinen T, Raj R. One-year outcome after aneurysmal subarachnoid hemorrhage in elderly patients. World Neurosurg. 2020. 143: e334-43 89. Wang Y, Cheng M, Liu S, Xie G, Liu L, Wu X. Shape related features of intracranial aneurysm are associated with rupture status in a large Chinese cohort. J Neurointerv Surg. 2022. 14: 252-6 90. Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: An updated meta-analysis. Stroke. 2007. 38: 1404-10 91. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10 92. Xiao W, Qi T, He S, Li Z, Ou S, Zhang G. Low wall shear stress is associated with local aneurysm wall enhancement on high-resolution MR vessel wall imaging. AJNR Am J Neuroradiol. 2018. 39: 2082-7 93. Yasui N, Magarisawa S, Suzuki A, Nishimura H, Okudera T, Abe T. Subarachnoid hemorrhage caused by previously diagnosed, previously unruptured intracranial aneurysms: A retrospective analysis of 25 cases. Neurosurgery. 1996. 39: 1096-100 94. Yi J, Zielinski D, Chen M. Cerebral aneurysm size before and after rupture: Case series and literature review. J Stroke Cerebrovasc Dis. 2016. 25: 1244-8 95. Zhou S, Dion PA, Rouleau GA. Genetics of intracranial aneurysms. Stroke. 2018. 49: 780-7