- Department of Neurosurgery, Faculty of Medicine Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
Correspondence Address:
Achmad Fahmi, Department of Neurosurgery, Faculty of Medicine Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_1245_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Agus Turchan, Achmad Fahmi, Achmad Kurniawan, Abdul Hafid Bajamal, Asra Fauzi, Tedy Apriawan. The change of serum and CSF BDNF level as a prognosis predictor in traumatic brain injury cases: A systematic review. 17-Jun-2022;13:250
How to cite this URL: Agus Turchan, Achmad Fahmi, Achmad Kurniawan, Abdul Hafid Bajamal, Asra Fauzi, Tedy Apriawan. The change of serum and CSF BDNF level as a prognosis predictor in traumatic brain injury cases: A systematic review. 17-Jun-2022;13:250. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11663
Abstract
Background: Mortality predictions following traumatic brain injury (TBI) may be improved by including genetic risk in addition to traditional prognostic variables. One promising target is the gene coding for brain-derived neurotrophic factor (BDNF), a ubiquitous neurotrophin important for neuronal survival and neurogenesis.
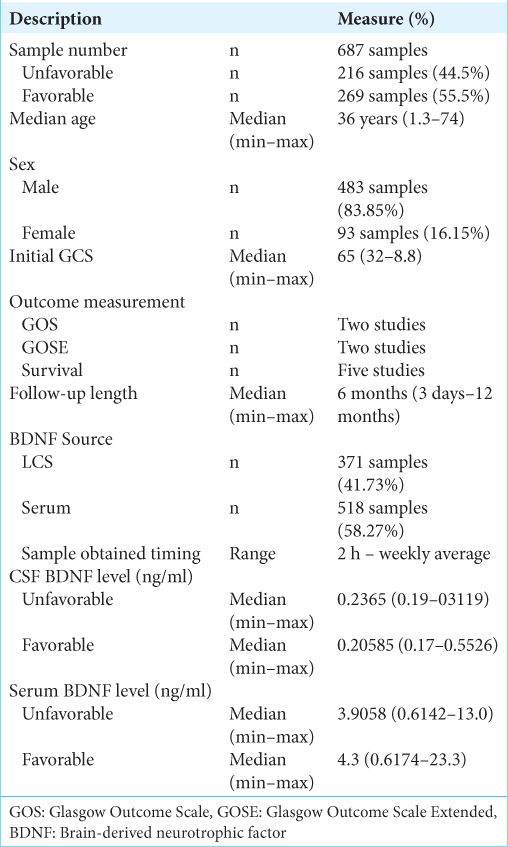
Methods: A total of seven publications pertaining to BDNF in the study of traumatic head injury were included and reviewed. The majority of patients were male, that is, 483 (83.85%) patients, compared to 93 (16.15%) female patients. The median length of follow-up was 6 months (3 days–12 months). Measurement of the patient’s initial condition was carried out by measuring the initial GCS of the patient at the time of admission across the five studies being 6.5. The median CSF BDNF levels in the unfavorable group being 0.2365 (0.19–0.3119) ng/ml, from favorable group which was 0.20585 (0.17–0.5526) ng/ml. The median serum BDNF level in the unfavorable group being 3.9058 (0.6142–13.0) ng/ml, from favorable group which was 4.3 (0.6174–23.3) ng/ml.
Results: Six studies reported on the sex distribution of patients, the majority of patients were male, that is, 483 (83.85%) patients, compared to 93 (16.15%) female patients. Six studies reported the number of patients per outcome group. The comparison of the number of patients in the two groups was quite balanced with the number of patients in the good group as many as 269 patients (55.5%) and the number of patients in the unfavorable group as many as 216 patients (44.5%). Measurement of the patient’s initial condition was carried out by measuring the patient’s initial GCS at the time of admission. It was reported in five studies, with the overall mean baseline GCS across five studies being 6.5 (3.2–8.8). Measurement of patient outcome was carried out by several methods, two studies used Glasgow Outcome Scale, Glasgow Outcome Scale Extended was used in two studies, and five studies used survival as a patient outcome measure. The patient’s BDNF level was measured in CSF and/or serum. A total of four studies measuring BDNF CSF levels and serum BDNF levels. Measurement of BDNF levels in TBI patients conducted on patients in seven literatures showed that there were differences in the trend of BDNF levels from CSF sources and serum sources. Measurement of CSF BDNF levels CSF BDNF levels was reported in two of the seven literatures, with the median CSF BDNF level in the unfavorable group being 0.2365 (0.19–0.3119) ng/ml. CSF BDNF levels were higher than the median in the preferred group, which was 0.20585 (0.17–0.5526) ng/ml. The results of the analysis from three other literatures stated that there was a tendency for lower CSF BDNF levels in the preferred group. Serum BDNF levels were reported in two of the seven literatures, with the median serum BDNF level in the unfavorable group being 3.9058 (0.6142–13.0) ng/ml. This serum BDNF level was lower than the median in the preferred group, which was 4.3 (0.6174–23.3) ng/ml. The results of the analysis of four literatures reporting serum BDNF stated that there was a tendency for lower serum BDNF levels in the poor group. A risk assessment of bias for each study was performed using ROBINS-I because all included studies were non-RCT studies. Overall the results of the risk of bias analysis were good, with the greatest risk of confounding bias and outcome bias.
Conclusion: Serum BDNF levels were found to be lower in the unfavorable group than in the favorable group. This is associated with an increase in autonomic function as well as a breakdown of the blood–brain barrier which causes a decrease in serum BDNF levels. Conversely, CSF BDNF levels were found to be higher in the unfavorable group than in the favorable group. This is associated with an increase in the breakdown of the blood–brain barrier which facilitates the transfer of serum BDNF to the brain, leading to an increase in CSF BDNF levels.
Keywords: Brain-derived neurotrophic factor, Brain injury, Outcome, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a medical condition that occurs as a result of traumatic events in the brain. TBI has become the main cause of mortality and disability in 2020, according to the World Health Organization, with over 10 million people impacted each year. Advanced age is a consistent determinant of TBI survival. Available data indicate that nearly 60% of TBI cases are caused by road traffic injuries worldwide; about 20–30% due to falls; 10% due to violence; and another 10% due to accidents at work and during sports. Recent literatures indicated that individuals who survive the acute phase after severe TBI have an increased risk of death during the postacute recovery phase and, on average, have a shorter life span compared to general population.[
Systemic biomarkers from the central nervous system (CNS) can provide information regarding the prediction of acute death after severe TBI. In addition, biomarkers describe TBI-specific pathology relevant to the molecular pathways they represent. While few studies have evaluated patterns of early biomarkers that predict mortality, less is known about how innate biological factors, such as genetics, interact with acute biomarkers to influence mortality.[
Brain-derived neurotrophic factor (BDNF), one of a group of neurotrophic proteins, is an autocrine factor that triggers cell development, differentiation, and regeneration. BDNF is also important for synaptic plasticity and regeneration of neuronal cells. BDNF has been known to have the effect of reducing the severity of secondary brain injury so that it can protect nerve cells and improve connections between these cells after trauma.[
BDNF, a neurotrophin expressed in the brain, is an attractive target for TBI intervention research because of its role in neuronal survival, neurogenesis, and plasticity. BDNF and its major receptors are found in various areas of the brain, including structures of the cortex, striatum, hypothalamus, septum, thalamus, and cerebellum. The highest levels of BDNF were found in the hippocampus structure.[
Although the BDNF is known to have a protective effect on the incidence of TBI in terms of protection of nerve cells, the exact mechanism is still not clearly understood. Therefore, this study was designed to study usefulness of measuring BDNF levels as prognosticator of TBI.
MATERIALS AND METHODS
Eligibility criteria
Any studies investigating BDNF in patient with TBI will be included in this study. Literature with the following design: clinical, prospective, retrospective, and observational cohort trials.
Type of outcome measures
Clinical outcome measured with:
Glasgow Outcome Scale (GOS) Glasgow Outcome Scale Extended (GOSE) Survival
Information sources
This systematic review was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.[
Search protocol
The study question was formed from the PICO. The authors used the following search keywords to search all trials registers and databases: (“Brain-derived Neurotrophic Factor” OR “BDNF” OR “Neurotrophic”) AND (“Traumatic Brain Injury” OR “TBI” OR “Brain Injury”).
Data collection and analysis
We screened all records on the title and abstract as a result of the search strategy. Three authors (AK, AMHT, and AT) independently assessed for inclusion all potential studies. The search results were first excluded based on the nonrelevancy of the titles followed with the abstracts. Non-English publications were automatically excluded from the study. Articles were then assessed by all authors for potentially eligible clinical, prospective, retrospective, and observational cohort trials. The reasons of exclusion were noted. Included studies are resumed in
Assessment of study quality and risk of bias in included studies
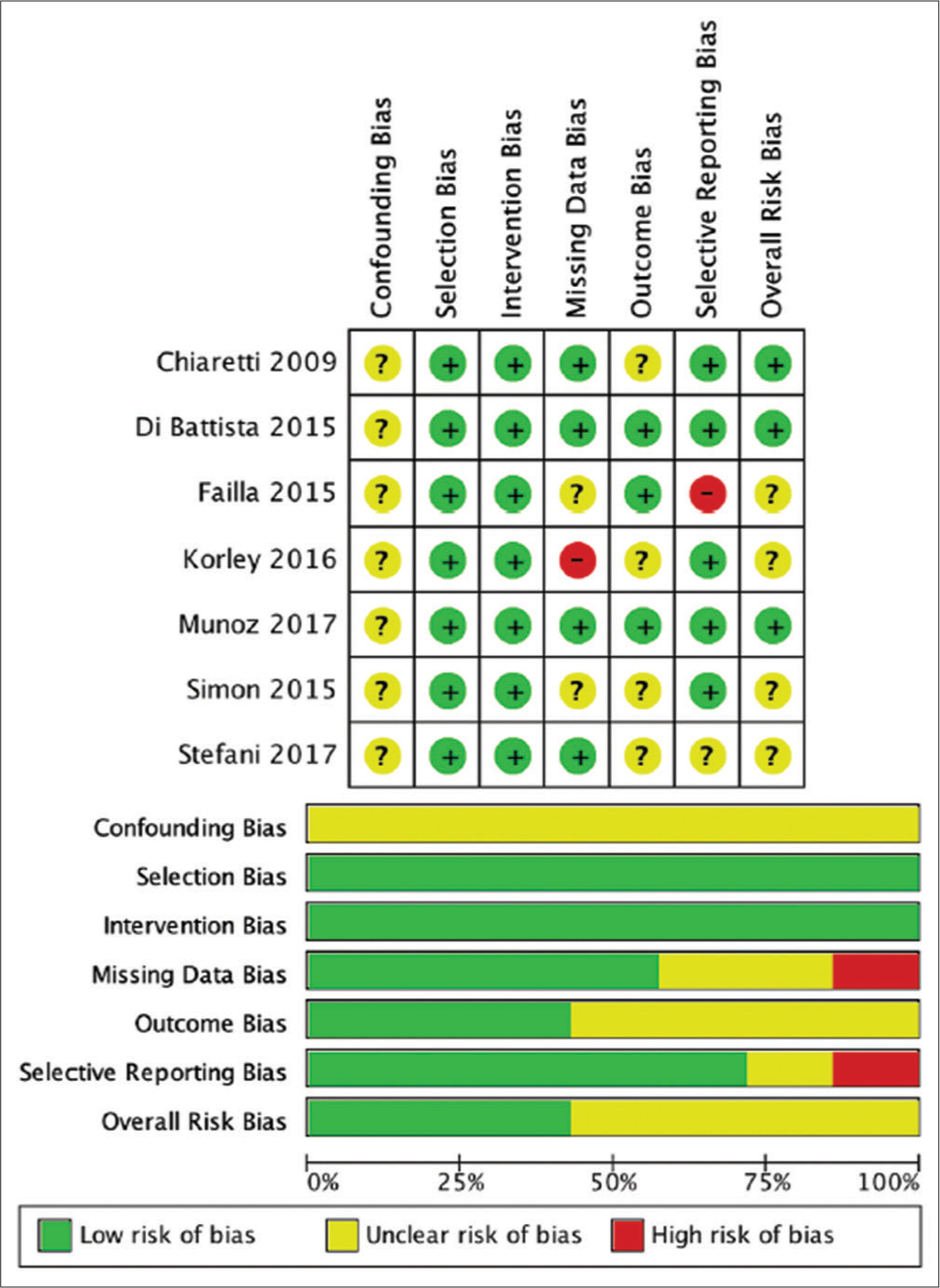
Review authors independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions for nonrandomized studies, called as Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) for nonrandomized studies and Risk of Bias 2 for randomized studies. Any disagreement was resolved by discussion with the fourth author. We summarized judgments in “Risk of bias” tables along with the characteristics of the included studies and interpreted the results of meta-analyses in light of the overall “Risk of bias” assessment.
RESULTS
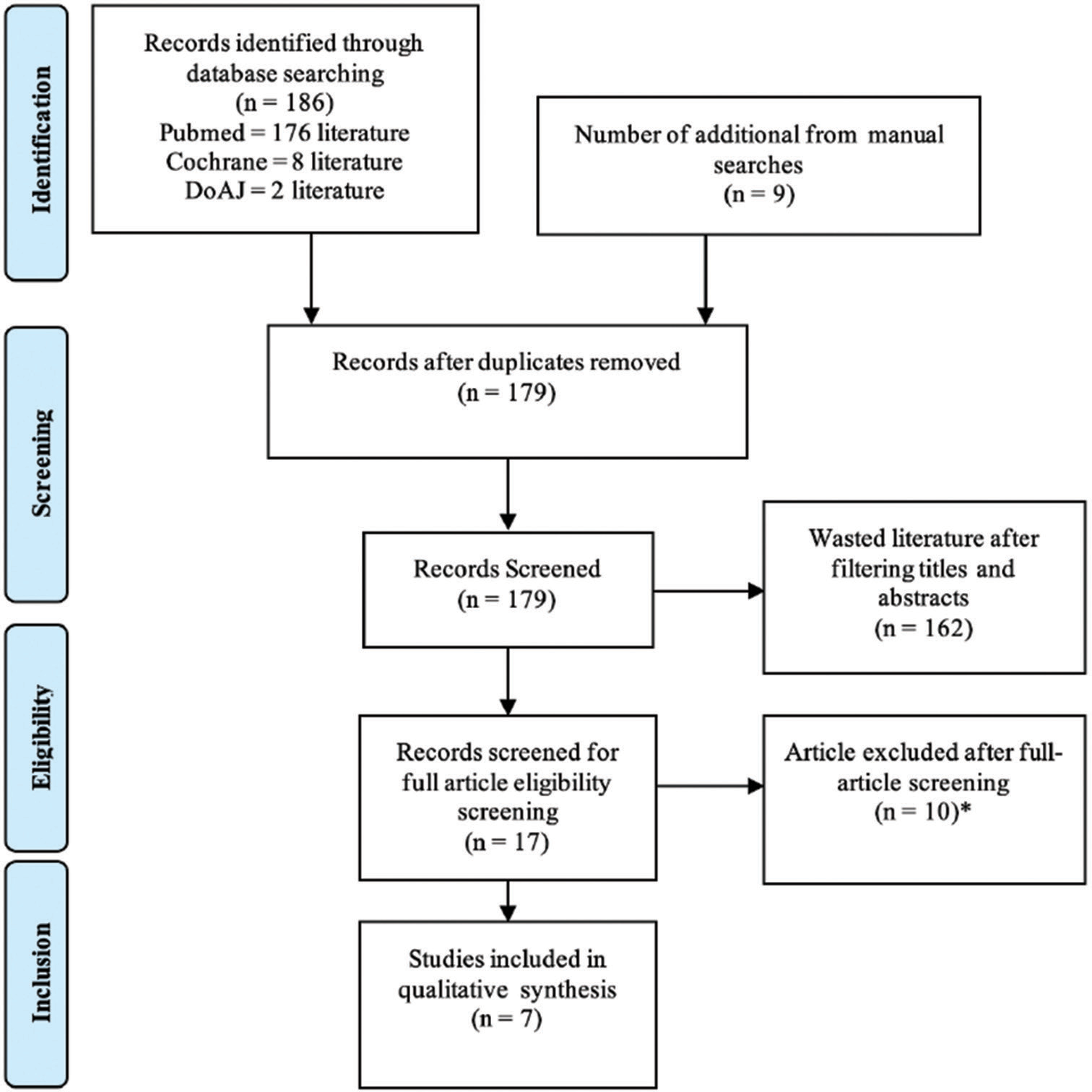
The literature search was conducted from multiple online databases. The online journal databases used in the search were PubMed (176 literature), Cochrane (eight literature), and DoAJ (two literature), and another nine studies were obtained by manual search, for a total of 195 studies. After the duplication screening, a total of 179 literatures were obtained then filtered through titles and abstracts and obtained 17 literatures. From these 17 literatures, the text was filtered thoroughly, and seven literatures were found that met the criteria. These seven were used to conduct a systematic review using qualitative data. The flowchart of the research literature search results is shown in
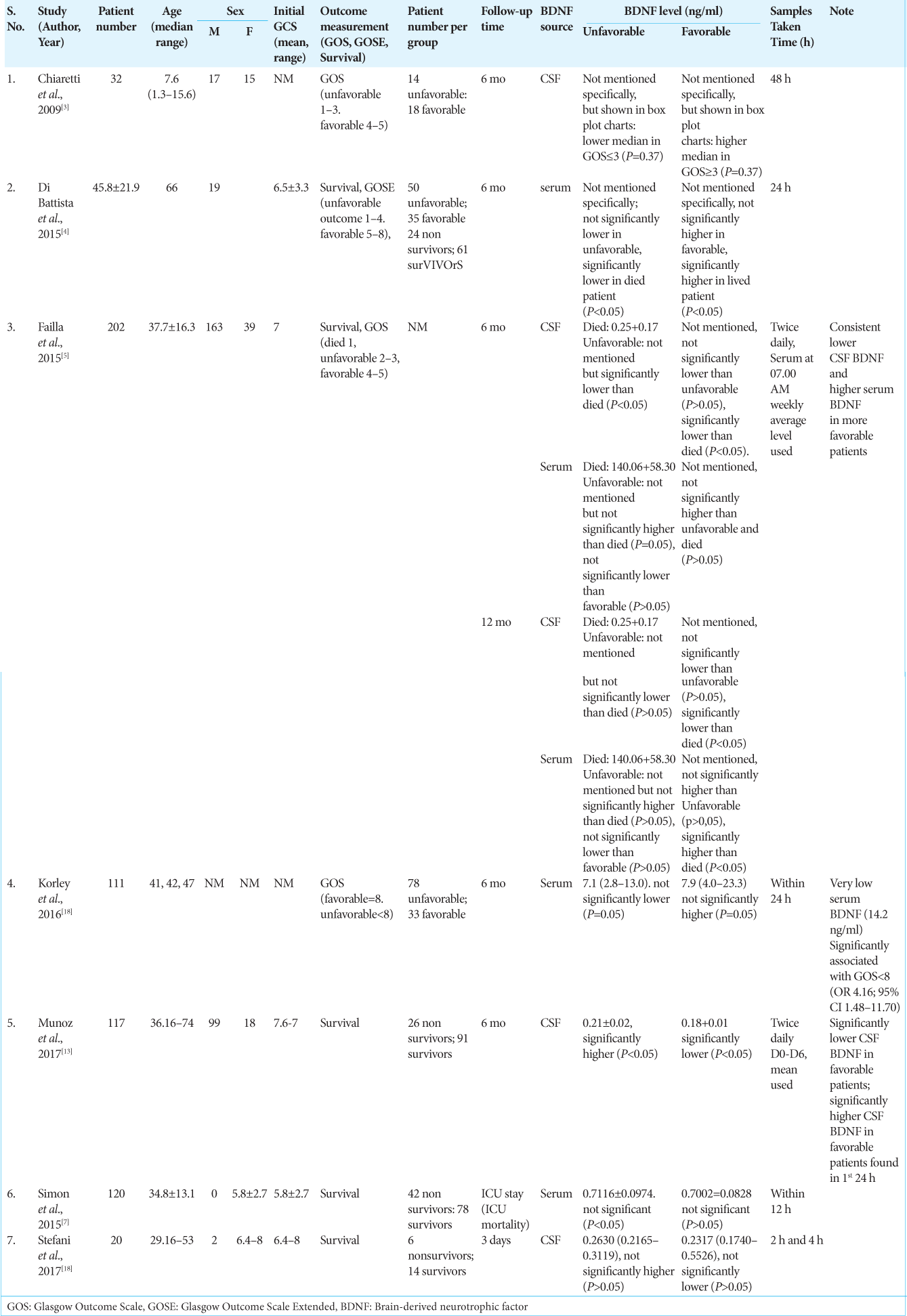
The authors summary of data from all seven studies included in the systematic review is shown in
Demographics
A total of seven literatures were included in our study. The total number of patients was 687 with a median age of 36 (1.3–74) years. Six studies reported on the sex distribution of patients, for a total sample of 576 patients. Of these six studies, the majority of patients were male, that is, 483 (83.85%) patients, compared to 93 (16.15%) female patients.
All patients were divided into two groups based on the final outcome, namely, favorable and unfavorable. A total of studies reported the number of patients per outcome group, for a total of 485 patients. There were 269 patients (55.5%) in the favorable group and 216 patients (44.5%) in the unfavorable group.
Measurement of initial condition and patient outcome
Measurement of the patient’s initial condition was carried out by measuring the initial GCS of the patient at the time of admission. The median baseline GCS was reported in five studies only, with the overall median baseline GCS across the five studies being 6.5 (3.2–8.8).
Measurement of patient outcomes was carried out by several methods and some studies used more than 1 outcome measure. Two studies used GOS as well as GOSE as a measure of patient outcome. Five studies used survival as a measure of patient outcome.
The length of follow-up in seven studies was reported with certainty in six studies, and the other one study measured the length of stay in the intensive care unit/ICU. The median length of follow-up was 6 months (3 days–12 months).
Patients’ BDNF levels were measured in CSF and/or serum. Failla et al.’s, 2015, study carried out measurements of both CSF and serum and was the only study to measure both.[
Demographic data and measurements of baseline conditions and patient outcomes in the literature included in this study are presented in a tabular summary in
Measurement of BDNF levels in TBI patients
Measurement of BDNF levels in TBI patients conducted on patients in the seven literatures showed a difference between the trend of BDNF levels from CSF sources and serum sources. Each of these will be described below.
Measurement of BDNF CSF levels
BDNF CSF levels were reported in two of seven literatures, with the median CSF BDNF levels in the unfavorable group being 0.2365 (0.19–0.3119) ng/ml. The CSF BDNF level was higher than the median in the favorable group, which was 0.20585 (0.17–0.5526) ng/ml. The trend of lower CSF BDNF levels in the favorable group compared to the unfavorable group was also seen in reports from all four literatures reporting CSF BDNF levels, except in the 2009 Chiaretti et al. study which reported that lower CSF BDNF levels in the unfavorable group, but the results this was not statistically significant (P = 0.37).[
The results of the analysis of three other literatures stated that there was a tendency for lower CSF BDNF levels in the favorable group. Studies by Failla et al. and Munoz et al. which obtained weekly average CSF BDNF levels showed significant results, meanwhile, a study by Stefani et al. taking CSF BDNF samples at 2 and 4 h posthospitalization demonstrated negligible findings.[
Measurement of serum BDNF level
Serum BDNF levels were reported in two of the seven literatures, with the median serum BDNF level in the unfavorable group being 3.9058 (0.6142–13.0) ng/ml. This serum BDNF level was lower than the median in the favorable group, which was 4.3 (0.6174–23.3) ng/ml.[
The results of the analysis of four literatures reporting serum BDNF stated that there was a tendency for lower serum BDNF levels in the unfavorable group. This result was not significant between the unfavorable group and the favorable group, but significantly lower serum BDNF levels were reported in the nonsurvival group compared to the survival group in the 2015 study by Di Battista et al.[
Risk of bias
The risk assessment of bias for each study was carried out using ROBINS-I because all included studies were non-RCT studies. Overall the results of the risk analysis of bias were good, with the greatest risk of confounding bias and outcome bias. The results of the risk assessment of bias with ROBINS-I are shown in
DISCUSSION
This study was conducted by means of a systematic literature search of all literature that report assessing patients’ BDNF levels and is associated with TBI patient outcomes. The literature study systematically produced seven literatures which were used for qualitative analysis. BDNF can be used to predict the outcome of TBI patients, both from serum BDNF and CSF BDNF. BDNF levels can even be used to assess long-term outcomes and mortality up to 12 months after trauma.[
Because this test is still relatively unpopular, the ability to check BDNF is still not evenly distributed and there is no definite level number associated with the outcome in TBI patients, so this test is not routinely performed. Several studies are also still debating the benefits of this examination.[
Demographics
The study of seven literatures resulted in a total of 687 patients. The median age obtained from this study was 36 years with the youngest age 1.3 years and the oldest 74 years. Six studies reported on the sex distribution of patients, for a total sample of 576 patients. Of these six studies, the majority of patients were male, that is, 483 (83.85%) patients, compared to 93 (16.15%) female patients. Various studies have revealed sex differences in TBI. They noted that the number of male patients was higher than that of women, due to the increased likelihood of injury in males, due to the high rates of traffic mobility and risky sports activities in males compared to females.[
All patients were generally divided into two groups based on the final outcome, namely, favorable and unfavorable. Six studies reported the number of patients per outcome group, for a total of 485.[
Confounding factors
Examination of BDNF levels to evaluate and estimate the outcome of TBI patients to date is an additional examination that has not been routinely performed. Therefore, there are several things that confound this situation. First, the source and timing of BDNF collection varies greatly, depending on the health facility. Second, the method of assessing BDNF levels also varies greatly, depending on each health facility. Finally, the time of patient follow-up and assessment of patient outcomes also differed between studies, making it difficult to interpret the data homogeneously. The author realizes that these three factors act as confounding factors, so the discussion of these factors is carried out in this study.
Measurement of the patient’s initial condition
Measurement of the patient’s initial condition was carried out by measuring the initial GCS of the patient at the time of admission. The median baseline GCS was reported in five studies, with the overall median baseline GCS across the five studies being 6.5 (3.2–8.8). This is in accordance with the theory that BDNF is indeed more valued in severe brain injury, considering that in cases of mild and moderate brain injury, the BDNF number is not much different between favorable and nonfavorable patients.[
Patient outcome measurement
Measurement of patient outcomes was carried out by several methods and some studies used more than 1 outcome measure.
Five studies used survival as a measure of patient outcome. Several studies show that indeed, BDNF is more significant for showing survival outcomes than differ favorable and unfavorable outcomes.[
Patient outcome follow-up
The length of follow-up in seven studies was reported with certainty in six studies, and the other one study measured the length of stay in the intensive care unit/ICU. The median length of follow-up was 6 months (3 days–12 months). It seemed that studies which had longer than 6-month median follow-up would likely to show significant outcome compared to the one with shorter median follow-up.[
Measurement of BDNF levels in patients
Patients’ BDNF levels were measured in CSF and/or serum. Failla et al.’s, 2015, study carried out measurements of both CSF and serum and were the only study to measure both. A total of four studies measured CSF BDNF levels and serum BDNF levels, with 371 patients (41.73%) having CSF BDNF measurements and 518 patients (58.27%) having serum BDNF measurements. Sample measurement time ranged from 2 h to 1 week with two studies using weekly average BDNF levels.[
Measurement of BDNF levels in TBI patients commonly uses CSF or serum samples. CSF BDNF levels tend to be low in favorable patients, and conversely, serum BDNF levels tend to be high in favorable patients.[
The results of various studies show an increase in serum BDNF levels in the early phase and a decrease after 24 h in the unfavorable group due to the acute stress response which initially causes an increase in serum BDNF in severe TBI conditions in the first 24 h. Furthermore, an increase in autonomic function causes activation of the HPA axis, leading to a decrease in serum BDNF levels after 24 h.[
Measurement of BDNF levels in TBI patients
Measurement of BDNF levels in TBI patients conducted on patients in the seven literatures showed a difference between the trend of BDNF levels from CSF sources and serum sources. There was a trend of higher serum BDNF levels in the more favorable group and, conversely, the trend of lower CSF BDNF levels in the unfavorable group.[
Measurement of CSF BDNF levels
BDNF CSF levels were reported in two of seven literatures, with the median CSF BDNF levels in the unfavorable group being 0.2365 (0.19–0.3119) ng/ml. This CSF BDNF level was higher than the median in the favorable group, which was 0.20585 (0.17–0.5526) ng/ml.[
The trend of lower CSF BDNF levels in the favorable group compared to the unfavorable group was also seen in reports from all four literatures reporting CSF BDNF levels, except for 2009 Chiaretti et al. study which reported on the contrary, lower CSF BDNF levels in the unfavorable group, but the results this was not statistically significant (P = 0.37).[
The results of the analysis of three other literatures stated that there was a tendency for lower CSF BDNF levels in the favorable group. Two studies from Failla et al. and Munoz et al. showed statistically significant results.[
This is consistent with the theory that after severe TBI, there is a more significant disruption of the blood–brain barrier, causing an acute transfer of serum BDNF to the brain after TBI, leading to a decrease in serum BDNF levels and an increase in CSF BDNF levels in unfavorable patients.[
The insignificance of the results in some studies could be due to the delay required for the transfer of serum BDNF to the CSF within 24 h posttraumatic. In the study of Munoz et al., where it was hypothesized that, before the first 24 h, CSF BDNF levels could be lower in the unfavorable group because it takes time to transit from serum BDNF to the brain acutely after TBI causing a decrease in serum BDNF levels and an increase in CSF BDNF levels in unfavorable patients after 24 h posttrauma.[
Measurement of serum BDNF level
Serum BDNF levels were recorded in two of the seven studies, with the unfavorable group having a median serum BDNF level of 3.9058 (0.6142–13.0) ng/ml. This blood BDNF level was lower than the favorable group’s median, which was 4.3 (0.6174–23.3) ng/ml.[
The pattern of decreased CSF BDNF levels in the unfavorable group compared to the favorable group was also evident in serum BDNF level findings from all four literatures. The results of the analysis of four literatures reporting serum BDNF stated that there was a tendency for lower serum BDNF levels in the unfavorable group. This result was not significant between the unfavorable group and the favorable group, but significantly lower serum BDNF levels were reported in the nonsurvival group compared to the survival group in 2015 Di Battista et al. study.[
The decrease in serum BDNF levels in the unfavorable group is thought to be due to an increase in autonomic function in patients with more severe conditions so that activation of the HPA axis causes a decrease in BDNF levels.[
The insignificance of the results of the included studies could be due to the premature sampling time, the majority of which were within the first 24 h. According to the literature study, there is a delay required for the transfer of serum BDNF to the CSF within 24 h after trauma. In addition, in acute conditions, before 24 h, the response to acute stress results in an increase in serum BDNF first before a decrease in serum BDNF then because the increase in autonomic function in patients with more severe conditions causes activation of the HPA axis and suppresses BDNF levels.[
CONCLUSION
Serum BDNF levels were found to be lower in the unfavorable group than in the favorable group. This is associated with an increase in autonomic function as well as a breakdown of the blood–brain barrier which causes a decrease in serum BDNF levels. Conversely, CSF BDNF levels were found to be higher in the unfavorable group than in the favorable group. This is associated with an increase in the breakdown of the blood– brain barrier which facilitates the transfer of serum BDNF to the brain, leading to an increase in CSF BDNF levels.
Yet, serum BDNF and CSF levels have the potential to be outcome predictors in brain injury patients, especially those with severe conditions. Specific measurement methods and timing are required for the use of BDNF levels as predictors that can be widely used in everyday clinical practice.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Blaha GR, Raghupathi R, Saatman KE, McIntosh TK. Brain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neuroscience. 2000. 99: 483-93
2. Brooks JC, Strauss DJ, Shavelle RM, Paculdo DR, Hammond FM, Harrison-Felix CL. Long-term disability and survival in traumatic brain injury: Results from the national institute on disability and rehabilitation research model systems. Arch Phys Med Rehabil. 2013. 94: 2203-9
3. Chiaretti A, Barone G, Riccardi R, Antonelli A, Pezzotti P, Genovese O. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology. 2009. 72: 609-16
4. Di Battista AP, Buonora JE, Rhind SG, Hutchison MG, Baker AJ, Rizoli SB. Blood biomarkers in moderate-to-severe traumatic brain injury: Potential utility of a multi-marker approach in characterizing outcome. Front Neurol. 2015. 6: 110
5. Failla MD, Conley YP, Wagner AK. Brain-derived neurotrophic factor (BDNF) in traumatic brain injury-related mortality: interrelationships between genetics and acute systemic and central nervous system BDNF profiles. Neurorehabil Neural Repair. 2016. 30: 83-93
6. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007. 22: 341-53
7. Kalish H, Phillips TM. Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. 878: 194-200
8. Korley FK, Diaz-Arrastia R, Wu AH, Yue JK, Manley GT, Sair HI. Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J Neurotrauma. 2016. 33: 215-25
9. Ma C, Wu X, Shen X, Yang Y, Chen Z, Sun X. Sex differences in traumatic brain injury: A multi-dimensional exploration in genes, hormones, cells, individuals, and society. Chin Neurosurg J. 2019. 5: 24
10. Martinowich K, Lu B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology. 2008. 33: 73-83
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
12. Munivenkatappa A, Agrawal A, Shukla DP, Kumaraswamy D, Devi BI. Traumatic brain injury: Does gender influence outcomes?. Int J Crit Illn Inj Sci. 2016. 6: 70-3
13. Munoz MJ, Kumar RG, Oh BM, Conley YP, Wang Z, Failla MD. Cerebrospinal fluid cortisol mediates brain-derived neurotrophic factor relationships to mortality after severe TBI: A prospective cohort study. Front Mol Neurosci. 2017. 10: 44
14. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998. 37: 1553-61
15. Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012. 1264: 49-63
16. Santarsieri M, Niyonkuru C, McCullough EH, Dobos JA, Dixon CE, Berga SL. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J Neurotrauma. 2014. 31: 699-712
17. Simon D, Nascimento RI, Filho EM, Bencke J, Regner A. Plasma brain-derived neurotrophic factor levels after severe traumatic brain injury. Brain Inj. 2016. 30: 23-8
18. Stefani MA, Modkovski R, Hansel G, Zimmer ER, Kopczynski A, Muller AP. Elevated glutamate and lactate predict brain death after severe head trauma. Ann Clin Transl Neurol. 2017. 4: 392-402
19. Wagner AK, McCullough EH, Niyonkuru C, Ozawa H, Loucks TL, Dobos JA. Acute serum hormone levels: Characterization and prognosis after severe traumatic brain injury. J Neurotrauma. 2011. 28: 871-88