- Department of Adult Neurosurgery, National Neuroscience Institute, King Fahad Medical City, Riyadh, Saudi Arabia.
Correspondence Address:
Othman T. Almutairi, Department of Adult Neurosurgery, National Neuroscience Institute, King Fahad Medical City, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_114_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nada Alnefaie, Othman T. Almutairi, Abdulrahman Y. Alturki, Mohammed Bafaquh. Bibliometric analysis of the top 100 most-cited articles in neurofibromatosis. 01-Jul-2022;13:282
How to cite this URL: Nada Alnefaie, Othman T. Almutairi, Abdulrahman Y. Alturki, Mohammed Bafaquh. Bibliometric analysis of the top 100 most-cited articles in neurofibromatosis. 01-Jul-2022;13:282. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11689
Abstract
Background: Neurofibromatosis (NF) is an umbrella term that refers to three distinct disease entities: NF Type 1, Type 2, and schwannomatosis. Here, we reviewed the scientific performance and the most influential publications on NF.
Methods: A keyword-based search was performed using the Scopus database. The top 100 articles were grouped based on NF types and the studied entities. The differences between the articles, authors, and journals were quantified based on certain parameters. Other parameters were collected for the complete citational analysis.
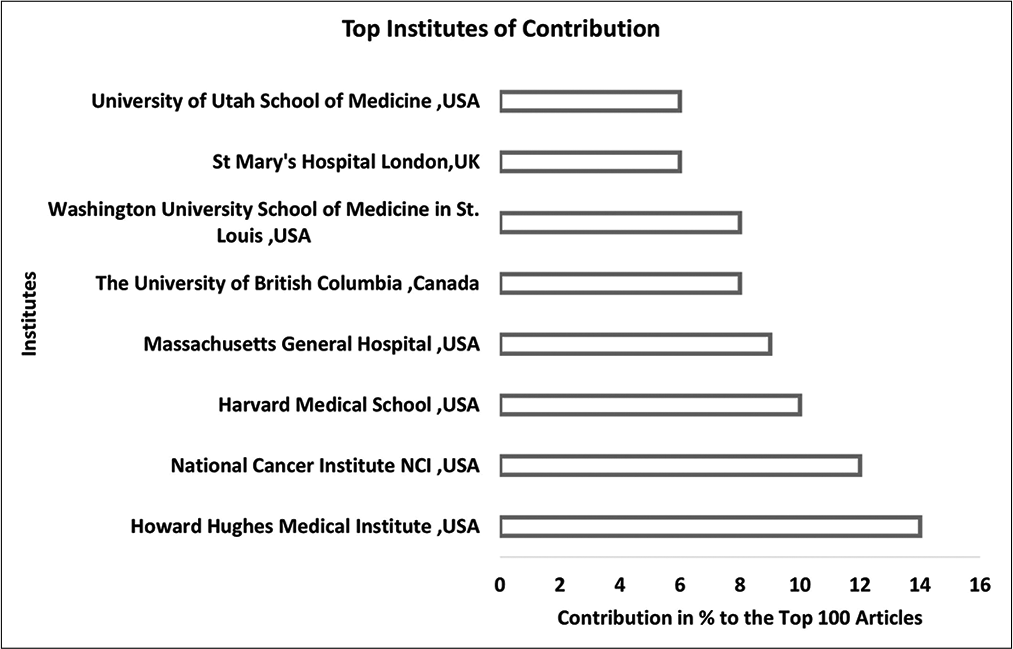
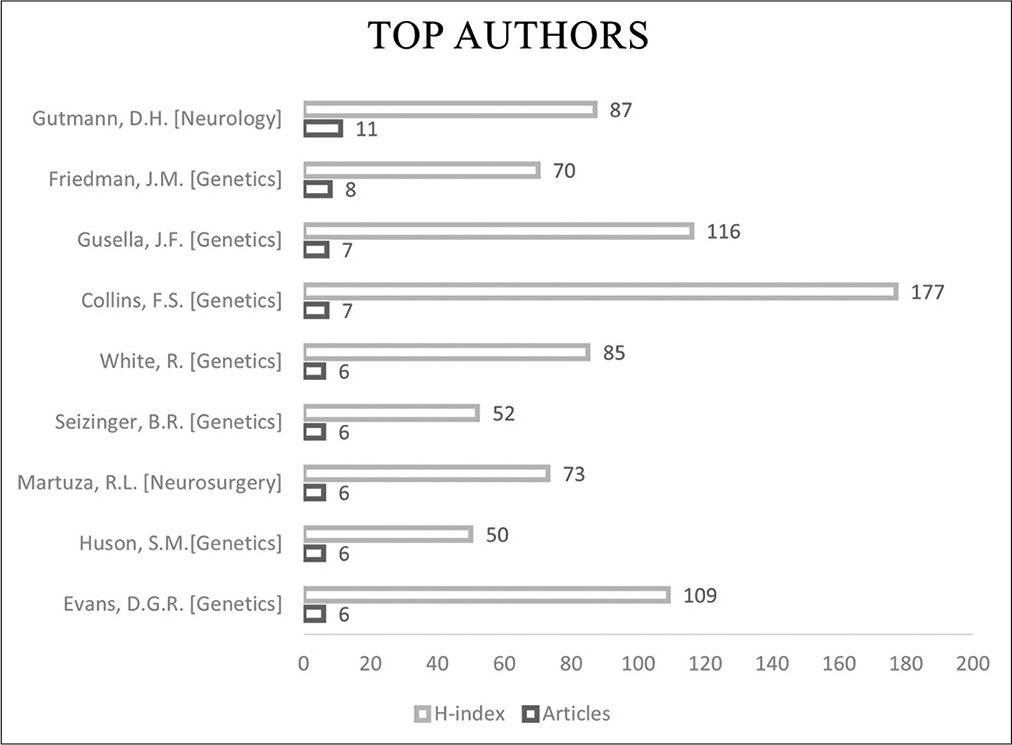
Results: The top 100 articles were published between 1961 and 2020. The most trending period of research was in the 1990s and articles studying the clinical aspect and the underlying genetic correlation made up 84% of all articles from the list. The United States of America (USA) had the highest number of contributions (69 articles, 69%). The top institute of contribution to the list was the Howard Hughes Medical Institute, USA (14 articles, 14%). Author-based analysis reveals that the neurologist D. H. Gutmann from St. Louis Children’s Hospital, USA, was the most active and authored 11 articles (11%) on the list.
Conclusion: The publication trends show that articles studying medical and surgical management were of little interest. The top 100 articles did not include any randomized control trials, and the highest level of evidence was obtained from reviews of pooled knowledge as well as population-based and longitudinal studies.
Keywords: Bibliometric, Neurocutaneous disorders, Neurofibroma, Neurofibromatosis, Von Recklinghausen disease
INTRODUCTION
The term neurofibromatosis (NF) was first introduced to the medical literature by Frederick von Recklinghausen in 1882 to characterize the structural relation and mutual involvement of neuromas and fibromas. [
A major type of NF and challenging differential of NF2 are schwannomatosis, also known as NF Type 3 (NF3).[
The management of NF involves many disciplines.[
Bibliometric analysis is an objective method to evaluate scientific publications’ performance in various fields.[
MATERIALS AND METHODS
A bibliometric keyword-based search using the Scopus database was utilized to perform a citation analysis of the top 100 most-cited articles on NF. A title-specific search using “NF,” “neurofibroma,” and “von Recklinghausen’s” was performed as search terms. The articles were rearranged based on their citation count (CC) in descending fashion. The top 100 articles were collected after excluding non-English, conference papers, erratum publications, and unpublished articles. The list of the top 100 articles on NF was categorized based on NF types to Type 1 and Type 2. Further subcategorization based on the studied entities of the top 100 articles yielded the following categories: (1) clinical, (2) genetics, (3) experimental, (4) histopathological, (5) radiological, (6) medical management, and (7) surgical management. The following parameters were considered when quantifying the difference between articles, authors, and journals: CC, citation per year (CY), Journal’s SCImago Journal Rank (SJR), Journal’s Source Normalized Impact Per Paper, Hirsch index (H-Index), and Journal Impact Factor (IF). The H-Index measures the productivity and the impact of an author by calculating the number of publications, he has been cited for at least the same number of times.[
RESULTS
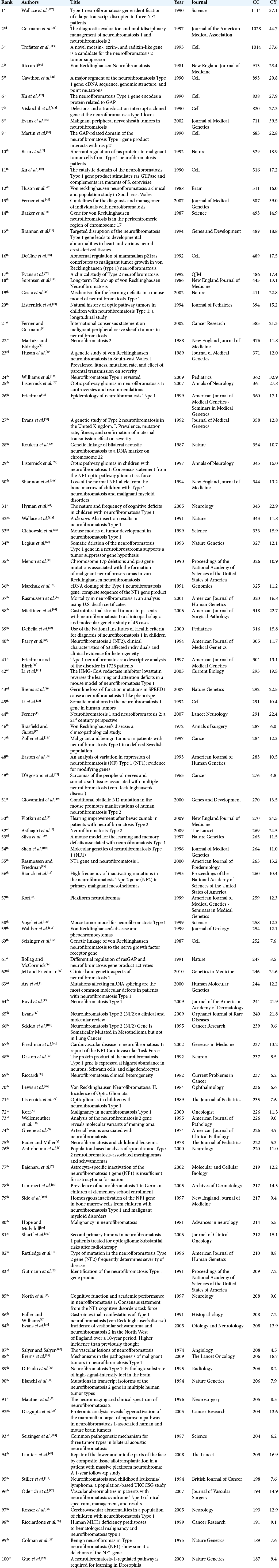
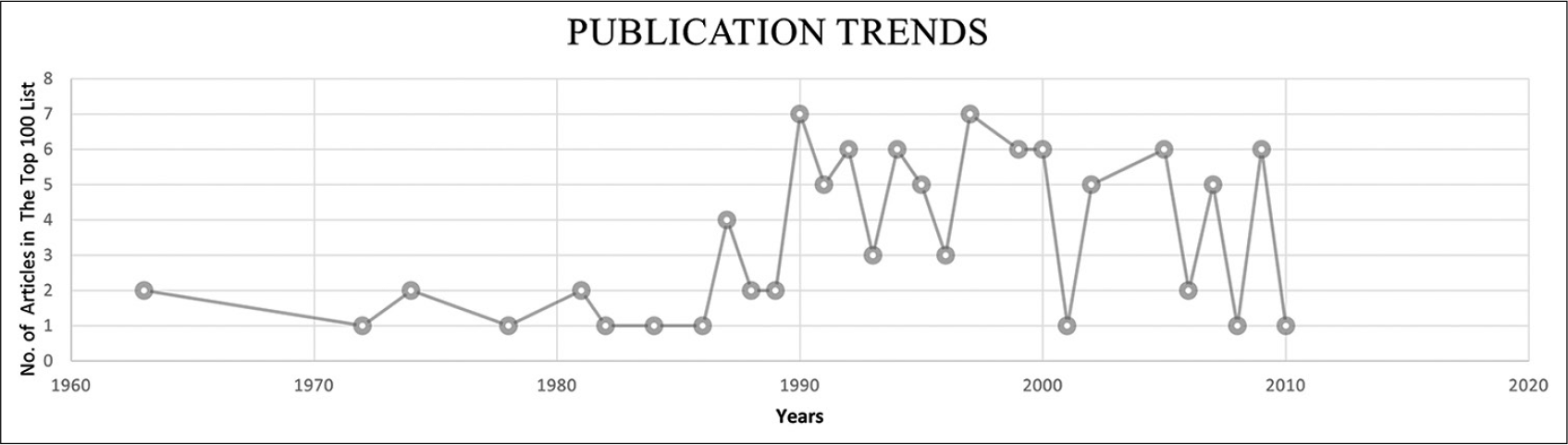
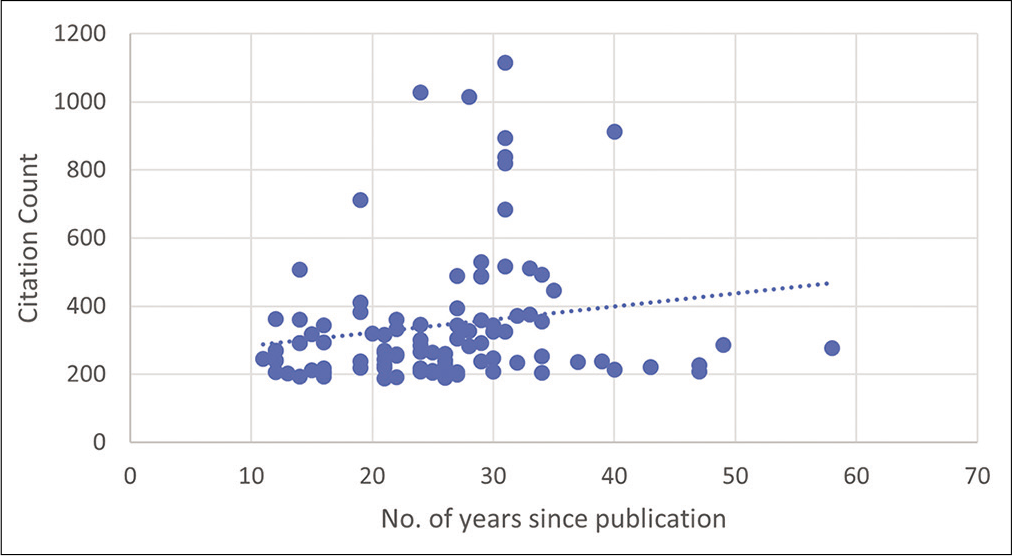
The keyword-based search identified 12,718 articles in which 8564 articles were included after excluding mismatched articles. The publication dates ranged from 1906 to 2020 in which the top 100 articles were published between 1961 and 2020. The top 100 articles CC accounted for 28,957 CCs; the self-citations rate was 7.56%, and the citations from books were 8.21% [
DISCUSSION
In the 1960s to 1986, NF publications focused on the pathological and clinical aspects. The association of NF to soft-tissue sarcoma, vascular lesions, and other types of malignancies was of great interest. Clinically, the literature offered updates on the current state of knowledge, established familiarity, and highlighted controversies and gaps for the future research.
In 1987–1997, the publications’ focus remarkably shifted toward discussing molecular pathogenesis and its biological behaviors. In this decade, the literature was dominated by genetic discoveries including population-based and prospective longitudinal studies. After 1997, clinical and experimental publications took over the trend with particular attention to the associated risks, epidemiology, and multidisciplinary management approach.
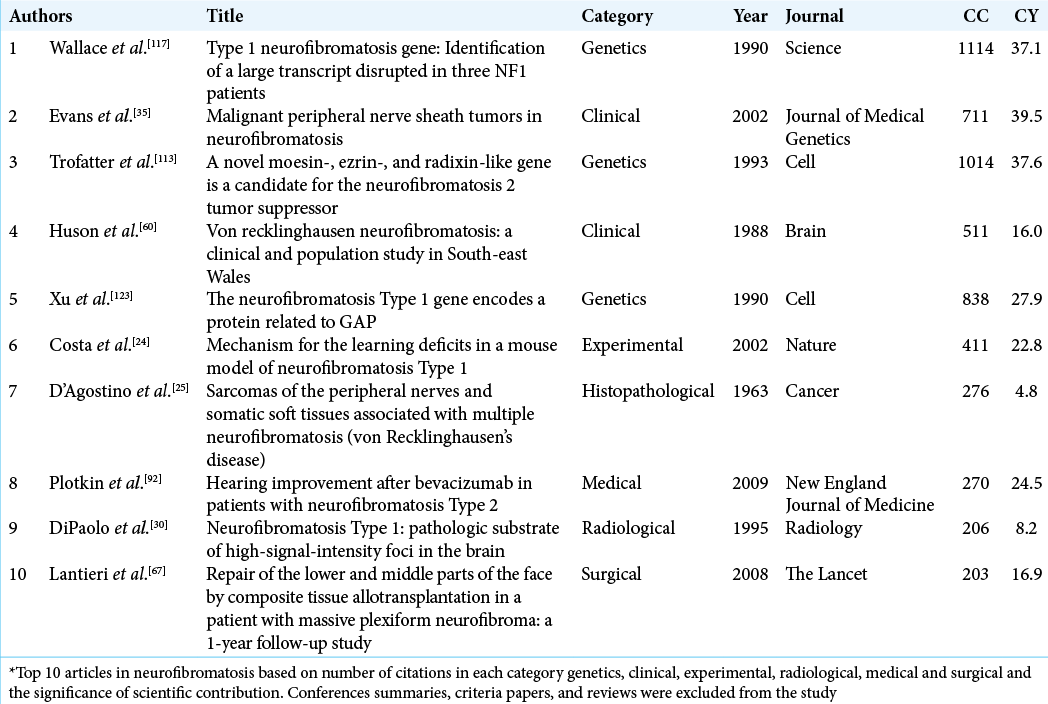
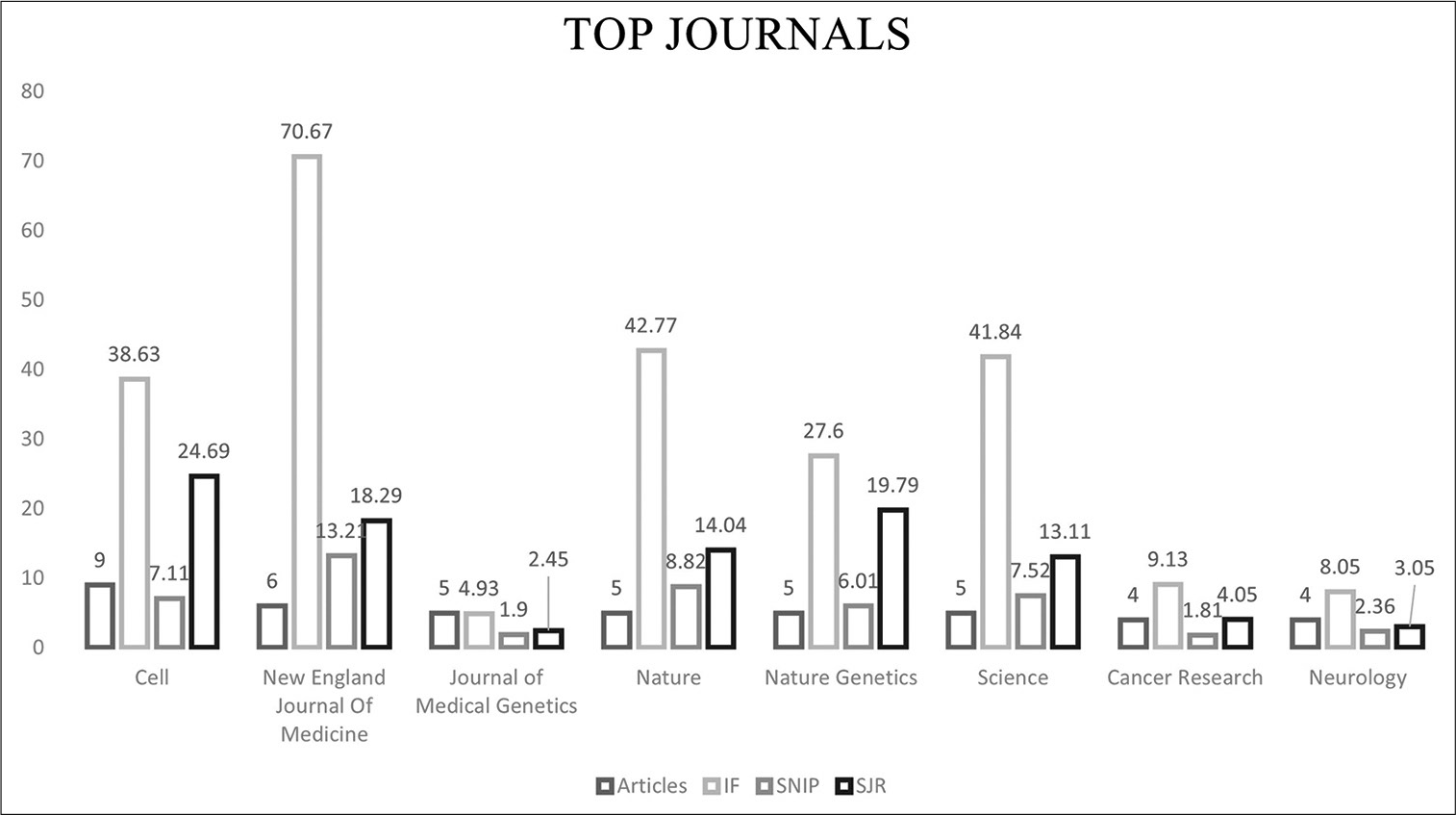
The top three highly cited articles in the NF field
The most-cited article involved the discovery of the gene responsible for NF1 (17q11.2) with 1114 CC and 37.1 CY. The article was published in Science under the genetic studies category. In 1990, Wallace et al. isolated and cloned the responsible NF1.[
The second most-cited article was published in the Journal of the American Medical Association and was a clinical study.
This had updated recommendations on NF diagnosis and management with 1028 CC and 44.7 CY. A thorough and systematic review of the NF literature between 1966 and 1996 was performed by the NF Clinical Care Advisory Board members and published in 1997.[
The third most-cited article was the identification, isolation, and cloning of the NF2 gene. The article was published in Cell under the genetic studies category with 1014 CC and 37.6 CY. In 1993, Trofatter et al. successfully identified the NF2 gene in which they also found it to be altered in meningiomas.[
NF publications by category
Clinical
In the late 20th century, publications were devoted to establishing a panoramic understanding of NF prevalence, natural history, specific clinical features, associated clinical disorders, and prognosis. In the early 21st century, the literature focused on the clinical category and slightly shifted to NF-associated risk of malignancy, management insights, and more in-depth knowledge about the disease epidemiology.[
The second highly cited article in this category (ranked fourth overall with 913 CC and 23.4 CY) was a review article of NF authored by Riccardi and published in the New England Journal of Medicine (1981). In his review, Riccardi offered comprehensive NF information sources including its clinical features, hypothesized pathogenesis, laboratory features, gaps in knowledge, and a recommended approach of management.[
Complementing Riccardi’s review and after NIH diagnostic criteria have been established (1987), Martuza and Eldridge published a review article of NF2 in the New England Journal of Medicine in 1988, (376 CC, 11.8 CY, ranked 22nd overall). The article added a valuable contribution to NF literature by distinguishing NF2 in light of the most updated diagnostic criteria.[
The third highly cited article in the clinical studies category was ranked 8th overall with 711 CC and 39.5 CY. A population-based and longitudinal study published in the Journal of Medical Genetics (2002) and was authored by Evan et al. In light of conflicting reports discussing rates of malignant peripheral nerve sheath tumors (MPNST) among NF1, this study investigated the survival rates and lifetime risk of MPNST in NF1 patients for 13 years (1984–1994). The data of MPNST and NF1 patients were obtained from regional cancer and genetic registries. The annual risk of MPNST in NF1 patients was 1.6/1000 and the estimated risk of a lifetime was 8–13%.[
Given the genetic basis and hereditary nature of NF of all types, population-based studies played a significant role in understanding the disease epidemiology and identifying patients at risk. The fourth highly cited article in the clinical studies’ category (ranked 12th overall) was a population-based study of NF1 authored by Huston et al. and published in Brain in 1988 (511 CC, 16 CY). The NF1 prevalence was 0.02% (1 in 5000). In addition to café-au-lait spots, axillary (64%) and groin (44%) freckling were standard associated features. Plexiform neurofibroma was identified in 32% of the cohort. The survey was conducted before the NIH diagnostic criteria of NF1; therefore, the criteria used did not include Lisch nodules, bony dysplasia, nor optic pathway glioma. However, Huston et al. selected the highly sensitive clinical features of NF1 and mentioned the recently established diagnostic criteria at the time, the paper was published.[
A similar population-based study on NF2 was performed (ranked 17th overall). The paper was published in the Quarterly Journal of Medicine, in 1992, and authored by Evan et al. (486 CC, 17.4CY). A comprehensive and regional method of data collection was conducted between 1989 and 1992. The reported age of onset was 21.57 years. Deafness was present in almost half of the cases; 80% was unilateral. Caféau-lait spots and nodular cutaneous manifestations were common findings at 43% and 68%.[
The eighth highly cited article in the clinical category (Ranked 20th overall) with 394 CC and 15.2 CY was authored by Listernick et al. and published in the Journal of Pediatrics (1994). A prospective and longitudinal study was conducted between 1985 and 1993. The primary goal was to determine the natural history of the optic pathway tumor (OPT) in children with NF. Repeated imaging and clinical exams in this subgroup of patients — with a median follow-up of 2.4 years and 3.4 years — confirmed a benign natural history with no evidence of either tumor growth or visual deterioration. Although a minority of cases progressively worsened, the study concluded that the risk of developing rapidly progressive OPT is at its greatest during the first 6 years of life, and regular ophthalmologic screening is recommended among this age group.[
Genetics
In the late 1980s, genes implicated in NF1 and NF2 were located on 17q11.2 and 22q1.2. Shortly after, the defective genes were identified, isolated, and cloned in multiple synergetic studies in 1990. These discoveries provided a groundwork for further studies to be performed on the encoded protein. Subsequently, and for over a decade, publications in the genetic category were directed toward a deeper understanding of the intermolecular interactions, molecular pathogenesis, phenotypic, genotypic correlation, and variations. [
Xu et al. authored the fourth highly cited article in the genetic studies category (Ranked sixth overall; Cell in 1990 with 838 CC and 27.9 CY). It described the discovery of the cloned NF1 gene peptide. The peptide had a 360 residue long sequence of amino acids similar to guanosine triphosphate hydrolase stimulatory proteins (GAPs).[
The sixth highly cited article in the genetic studies (ranked ninth overall) discovered a critical intermolecular interaction of the NF1 gene-encoded peptide. The study was authored by Martin et al. and published in Cell in 1990 with 683 CC and 22.8 CY. The GAP domain of the NF1 peptide enhanced the intrinsic GTPase activity of the intercellular P21ras protein. This finding hypothesized the encoded protein’s role in down-regulating and controlling cellular proliferation — a regular function that might have been impaired because of a mutated NF1 gene.[
A population-based genetic study was performed in 1992 to complement the previous clinical study of NF2.[
The mutations implicated in severe NF2 were investigated in another study. Frame shift-deletion/insertion mutations (i.e., protein-truncating mutations) were found to be associated with severe forms. Nevertheless, missense mutations were more likely to cause milder NF2 manifestations. These findings were reported by Ruttledge et al. and published in the American Journal of Human Genetics in 1996 (ranked 82nd overall, 210 CC, 8.8 CY).[
Experimental
Eight articles were identified in this category. All articles were published between the late 1990s and mid-2000s. Two major clinical problems were investigated in mice models. The first was learning difficulties and cognitive deficits in NF1. The second was tumor development in NF1 and NF2.[
The reported rates of cognitive deficits and learning disabilities in children with NF1 are higher than their regular counterparts. This domain was investigated by the highly cited article in the experimental studies category (Ranked 19th overall) on mice models. The article was authored by Costa et al. and published in Nature (2002; 411 CC and 22.8 CY). The experiment was performed on mice with an induced NF1 heterozygous null mutation. The mice carrying this mutated gene expressed learning deficits that were responsive to genetic and pharmacologic suppression of Ras activity and, subsequently, the implicated increased GABA mediated inhibition. This experiment postulated that learning disabilities in NF1 affected individuals and might be reversed through targeting this molecular pathway.[
Tumor development in NF2 was investigated in the fourth highly cited article in this category (ranked 51st overall). The study was authored by Giovannini et al. and published in Genes and Development (2000; 270 CC and 13.5 CY). The experiment used on mice models to show that schwannoma development requires two mutated alleles to express this phenotype. The mutated NF2 gene is also involved in the development of multiple neurocristopathies.[
Histopathological
D’Agostino et al. authored the highest cited article in histopathological studies (ranked 49th overall). Furthermore, it published in Cancer (1963; 276 CC and 4.8 CY). A retrospective and descriptive report of clinical course and histopathological findings of 21 patients with NF1. The report was one of earliest articles that detailed the cases’ general profile, clinical manifestations, pathological findings, treatment, and clinical course. The high number of citations could be contributed to two reasons. First is the noticeable trending period of research investigating the association of NF to soft-tissue sarcoma and other malignancies between 1960s and 1986. Second is the possible correlation between the publication date and the number of citations.[
Radiological
In the radiological studies category, two studies were in the top 100 list and were ranked 89th and 91st overall. The first paper (ranked 89th overall) was authored by Dipaolo et al. and published in Journal of Radiology (1995) with 206 CC and 8.2 CY. Brain autopsy findings in three NF1 patients were correlated with the findings on T2-weighted images.[
Medical management
In the medical management category, only one study was in the top 100 highest cited articles in NF (ranked 50th overall). The article was authored by Plotkin et al. and published in New England Journal of Medicine in 2009 with 270 CC and 24.5 CY. In this study, immunohistochemical analysis of the proportion of receptors expressed in NF2 vestibular schwannoma samples and sporadic schwannomas as controls were performed. Vascular endothelial growth factor (VEGF) receptors were found to be expressed in all NF2 vestibular schwannomas (21/21) and VEGFR-2 in 32% (7/21). Patients who were poor candidates for the standard treatment were offered bevacizumab — an anti-VEGF antibody. Among the patients’ treated, an imaging response was seen in 60% of subjects (6/10). Clinically, a hearing response was evident in 57% (4/7).[
Surgical management
The surgical management of a 29-year-male with diffuse facial plexiform neurofibroma who underwent composite tissue allograft facial transplantation was detailed by Lantieri et al. and published in The Lancet in 2008 (94th overall) with 203 CC and 16.9 CY. The article addressed surgical management and the postoperative course including immunosuppressive therapy and graft rejection monitoring. The benefits overweigh the risks in this case, which made the allotransplantation a possible treatment option in similar selected cases.[
Limitations and strengths
The top 100 articles were extracted from a single database, which could have added bias to the data presented. In addition, the correlation test performed showed that the older a published article is the more its citated. A positive correlation of 0.2 is, however, weak which indicates the presence of other factors that could have influenced the number of citations.
Implications
We provided a historical reference with a recognized pattern for the future research in the field of NF. If complemented with reviews from expertise, bibliometric studies can be utilized to predict future research directions and identify gaps in evidence.
CONCLUSION
We have presented the top 100 most influential publications on NF. Most articles addressed clinical and genetic aspects. In addition to extensive surveys and prospective and longitudinal studies, multidisciplinary statements produced from pooled evidence and expertise provided the highest level of evidence in these articles.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We want to thank the American Manuscript Editors for English language editing (www.americanmanuscripteditors.com).
References
1. Alfaifi A, AlMutairi O, Allhaidan M, Alsaleh S, Ajlan A. The top 50 most-cited articles on acoustic neuroma. World Neurosurg. 2018. 111: e454-64
2. Almutairi O, Albakr A, Al-Habib A, Ajlan A. The top-100 most-cited articles on meningioma. World Neurosurg. 2017. 107: 1025-32.e5
3. Antinheimo J, Sankila R, Carpén O, Pukkala E, Sainio M, Jääskeläinen J. Population-based analysis of sporadic and Type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000. 54: 71-6
4. Ars E, Serra E, García J, Kruyer H, Gaona A, Lázaro C. Mutations affecting mRNA splicing are the most common molecular defects in patients with neurofibromatosis Type 1. Hum Mol Genet. 2000. 9: 237-47
5. Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z. Neurofibromatosis Type 2. Lancet. 2009. 373: 1974-86
6. Bader JL, Miller RW. Neurofibromatosis and childhood leukemia. J Pediatr. 1978. 92: 925-9
7. Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002. 22: 5100-13
8. Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987. 236: 1100-2
9. Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from Type 1 neurofibromatosis patients. Nature. 1992. 356: 713-5
10. Belter CW. Bibliometric indicators: Opportunities and limits. J Med Libr Assoc. 2015. 103: 219-21
11. Bianchi AB, Hara T, Ramesh V, Gao J, Klein-Szanto AJ, Morin F. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994. 6: 185-92
12. Bianchi AB, Mitsunaga SI, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B. High frequency of inactivating mutations in the neurofibromatosis Type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A. 1995. 92: 10854-8
13. Blakeley JO, Ye X, Duda DG, Halpin CF, Bergner AL, Muzikansky A. Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis Type 2-associated vestibular schwannomas. J Clin Oncol. 2016. 34: 1669-75
14. Bollag G, McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature. 1991. 351: 576-9
15. Boyd KP, Korf BR, Theos A. Neurofibromatosis Type 1. J Am Acad Dermatol. 2009. 61: 1-14
16. Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW. Targeted disruption of the neurofibromatosis Type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994. 8: 1019-29
17. Brasfield RD, Das Gupta TK. Von Recklinghausen’s disease: A clinicopathological study. Ann Surg. 1972. 175: 86-104
18. Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis Type 1. Lancet Oncol. 2009. 10: 508-15
19. Brems H, Chmara M, Sahbatou M, Denayer E, Taniguchi K, Kato R. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet. 2007. 39: 1120-6
20. Brosius S. A history of von Recklinghausen’s NF1. J Hist Neurosci. 2010. 19: 333-48
21. Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, Stevens J. A major segment of the neurofibromatosis Type 1 gene: CDNA sequence, genomic structure, and point mutations. Cell. 1990. 62: 193-201
22. Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME. Mouse models of tumor development in neurofibromatosis Type 1. Science. 1999. 286: 2172-6
23. Colman SD, Williams CA, Wallace MR. Benign neurofibromas in Type 1 neurofibromatosis (NF1) show somatic deletions of the NF1 gene. Nat Genet. 1995. 11: 90-2
24. Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M. Mechanism for the learning deficits in a mouse model of neurofibromatosis Type 1. Nature. 2002. 415: 526-30
25. D’Agostino AN, Soule EH, Miller RH. Sarcomas of the peripheral nerves and somatic soft tissues associated with multiple neurofibromatosis (Von Recklinghausen’s disease). Cancer. 1963. 16: 1015-27
26. Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005. 65: 2755-60
27. Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N. The protein product of the neurofibromatosis Type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron. 1992. 8: 415-28
28. DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000. 105: 608-14
29. DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (Type 1) neurofibromatosis. Cell. 1992. 69: 265-73
30. DiPaolo DP, Zimmerman RA, Rorke LB, Zackai EH, Bilaniuk LT, Yachnis AT. Neurofibromatosis Type 1: Pathologic substrate of high-signal-intensity foci in the brain. Radiology. 1995. 195: 721-4
31. Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A. Activity of selumetinib in neurofibromatosis Type 1-related plexiform neurofibromas. N Engl J Med. 2016. 375: 2550-60
32. Easton DF, Ponder MA, Huson SM, Ponder BA. An analysis of variation in expression of neurofibromatosis (NF) Type 1 (NF1): Evidence for modifying genes. Am J Hum Genet. 1993. 53: 305-13
33. Elarjani T, Almutairi OT, Alhussinan MA, Alnefaie N, Alzhrani G, Bafaquh M. Bibliometric analysis of top 100 most cited articles on intraventricular hemorrhage. World Neurosurg. 2020. 144: e264-76
34. Evans D, Howard E, Giblin C, Clancy T, Spencer H, Huson S. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am J Med Genet Part A. 2010. 152: 327-32
35. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002. 39: 311-4
36. Evans DG, Bowers NL, Tobi S, Hartley C, Wallace AJ, King AT. Schwannomatosis: A genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018. 89: 1215-9
37. Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V. A clinical study of Type 2 neurofibromatosis. Q J Med. 1992. 84: 603-18
38. Evans DG, Huson SM, Donnai D, Neary W, Blair V, Teare D. A genetic study of Type 2 neurofibromatosis in the United Kingdom. I Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992. 29: 841-6
39. Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: Higher incidence than previously thought. Otol Neurotol. 2005. 26: 93-7
40. Evans DG. Neurofibromatosis Type 2 (NF2): A clinical and molecular review. Orphanet J Rare Dis. 2009. 4: 16
41. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002. 62: 1573-7
42. Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007. 44: 81-8
43. Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007. 6: 340-51
44. Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE. Cardiovascular disease in neurofibromatosis 1: Report of the NF1 cardiovascular task force. Genet Med. 2002. 4: 105-11
45. Friedman JM, Birch PH. Type 1 neurofibromatosis: A descriptive analysis of the disorder in 1,728 patients. Am J Med Genet. 1997. 70: 138-43
46. Friedman JM. Epidemiology of neurofibromatosis Type 1. Am J Med Genet. 1999. 89: 1-6
47. Fuller CE, Williams GT. Gastrointestinal manifestations of Type 1 neurofibromatosis (von Recklinghausen’s disease). Histopathology. 1991. 19: 1-11
48. Garfield E. The history and meaning of the journal impact factor. JAMA. 2006. 295: 90-3
49. Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis Type 2. Genes Dev. 2000. 14: 1617-30
50. Greene JF, Fitzwater JE, Burgess J. Arterial lesions associated with neurofibromatosis. Am J Clin Pathol. 1974. 62: 481-7
51. Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020. 382: 1430-42
52. Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000. 403: 895-8
53. Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997. 278: 51-7
54. Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013. 22: 443-62
55. Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis Type 1 gene product. Proc Natl Acad Sci U S A. 1991. 88: 9658-62
56. Hadfield KD, Newman WG, Bowers NL, Wallace A, Bolger C, Colley A. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008. 45: 332-9
57. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005. 102: 16569-72
58. Hope DG, Mulvihill JJ. Malignancy in neurofibromatosis. Adv Neurol. 1981. 29: 33-56
59. Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989. 26: 704-11
60. Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. A clinical and population study in SouthEast Wales. Brain. 1988. 111: 1355-81
61. Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis Type 1. Neurology. 2005. 65: 1037-44
62. Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010. 12: 1-11
63. Karajannis MA, Legault G, Hagiwara M, Ballas MS, Brown K, Nusbaum AO. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis Type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012. 14: 1163-70
64. Korf BR. Malignancy in neurofibromatosis Type 1. Oncologist. 2000. 5: 477-85
65. Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999. 89: 31-7
66. Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005. 141: 71-4
67. Lantieri L, Meningaud JP, Grimbert P, Bellivier F, Lefaucheur JP, Ortonne N. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: A 1-year follow-up study. Lancet. 2008. 372: 639-45
68. Legius E, Marchuk DA, Collins FS, Glover TW. Somatic deletion of the neurofibromatosis Type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat Genet. 1993. 3: 122-6
69. Lewis RA, Gerson LP, Axelson KA, Riccardi VM, Whitford RP. von Recklinghausen neurofibromatosis. II Incidence of optic gliomata. Ophthalmology. 1984. 91: 929-35
70. Li KL, Djoukhadar I, Zhu X, Zhao S, Lloyd S, McCabe M. Vascular biomarkers derived from dynamic contrast-enhanced MRI predict response of vestibular schwannoma to antiangiogenic therapy in Type 2 neurofibromatosis. Neuro Oncol. 2016. 18: 275-82
71. Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis Type 1. Curr Biol. 2005. 15: 1961-7
72. Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992. 69: 275-81
73. Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis Type 1: A longitudinal study. J Pediatr. 1994. 125: 63-6
74. Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis Type 1. J Pediatr. 1989. 114: 788-92
75. Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: Controversies and recommendations. Ann Neurol. 2007. 61: 189-98
76. Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997. 41: 143-9
77. MacCollin M, Chiocca EA, Evans DG, Friedman JM, Horvitz R, Jaramillo D. Diagnostic criteria for schwannomatosis. Neurology. 2005. 64: 1838-45
78. Marchuk DA, Saulino AM, Tavakkol R, Swaroop M, Wallace MR, Andersen LB. cDNA cloning of the Type 1 neurofibromatosis gene: Complete sequence of the NF1 gene product. Genomics. 1991. 11: 931-40
79. Markham A, Keam SJ. Selumetinib: First Approval. Drugs. 2020. 80: 931-7
80. Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H. The GAP-related domain of the neurofibromatosis Type 1 gene product interacts with ras p21. Cell. 1990. 63: 843-9
81. Martuza RL, Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med. 1988. 318: 684-8
82. Mautner VF, Lindenau M, Baser ME, Hazim W, Tatagiba M, Haase W. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996. 38: 880-5
83. Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990. 87: 5435-9
84. Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: A clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006. 30: 90-6
85. 86. North KN, Riccardi V, Samango-Sprouse C, Ferner R, Moore B, Legius E. Cognitive function and academic performance in neurofibromatosis. 1 Consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997. 48: 1121-7 87. Oderich GS, Sullivan TM, Bower TC, Gloviczki P, Miller DV, Babovic-Vuksanovic D. Vascular abnormalities in patients with neurofibromatosis syndrome Type I: Clinical spectrum, management, and results. J Vasc Surg. 2007. 46: 475-84 88. Parry DM, Eldridge R, Kaiser-Kupfer MI, Bouzas EA, Pikus A, Patronas N. Neurofibromatosis 2 (NF2): Clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994. 52: 450-61 89. Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014. 46: 182-7 90. Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010. 31: 1135-43 91. Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis Type 2: A retrospective review of 31 patients. Otol Neurotol. 2012. 33: 1046-52 92. Plotkin SR, Stemmer-Rachamimov AO, Barker FG, Halpin C, Padera TP, Tyrrell A. Hearing improvement after bevacizumab in patients with neurofibromatosis Type 2. N Engl J Med. 2009. 361: 358-67 93. Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000. 151: 33-40 94. Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: An analysis using U.S. death certificates. Am J Hum Genet. 2001. 68: 1110-8 95. Riccardi VM. Neurofibromatosis: Clinical heterogeneity. Curr Probl Cancer. 1982. 7: 1-34 96. Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981. 305: 1617-27 97. Ricciardone MD, Özçelik T, Cevher B, Özdağ H, Tuncer M, Gürgey A. Human MLH1 deficiency predisposes to hematological maligancy and neurofibromatosis Type 1. Cancer Res. 1999. 59: 290-3 98. Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis Type 1. Neurology. 2005. 64: 553-5 99. Rouleau GA, Wertelecki W, Haines JL, Hobbs WJ, Trofatter JA, Seizinger BR. Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 1987. 329: 246-8 100. Ruggieri M, Praticò AD, Caltabiano R, Polizzi A. Early history of the different forms of neurofibromatosis from ancient Egypt to the British Empire and beyond: First descriptions, medical curiosities, misconceptions, landmarks, and the persons behind the syndromes. Am J Med Genet A. 2018. 176: 515-50 101. Ruttledge MH, Andermann AA, Phelan CM, Claudio JO, Han FY, Chretien N. Type of mutation in the neurofibromatosis Type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet. 1996. 59: 331-42 102. Salyer WR, Salyer DC. The vascular lesions of neurofibromatosis. Angiology. 1974. 25: 510-9 103. Seizinger BR, Rouleau G, Ozelius LJ, Lane AH, St George-Hyslop P, Huson S. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987. 236: 317-9 104. Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987. 49: 589-94 105. Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF. Neurofibromatosis Type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995. 55: 1227-31 106. Shannon KM, O’Connell P, Martin GA, Paderanga D, Olson K, Dinndorf P. Loss of the normal NF1 allele from the bone marrow of children with Type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med. 1994. 330: 597-601 107. Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: Substantial risks after radiotherapy. J Clin Oncol. 2006. 24: 2570-5 108. Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis Type 1 (NF1). J Med Genet. 1996. 33: 2-17 109. Side L, Taylor B, Cayouette M, Conner E, Thompson P, Luce M. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis Type 1 and malignant myeloid disorders. N Engl J Med. 1997. 336: 1713-20 110. Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D. A mouse model for the learning and memory deficits associated with neurofibromatosis Type I. Nat Genet. 1997. 15: 281-4 111. Sørensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986. 314: 1010-5 112. Stiller CA, Chessells JM, Fitchett M. Neurofibromatosis and childhood leukaemia/lymphoma: A population-based UKCCSG study. Br J Cancer. 1994. 70: 969-72 113. Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993. 72: 791-800 114. Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis Type 1 locus. Cell. 1990. 62: 187-92 115. Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis Type 1. Science. 1999. 286: 2176-9 116. Wallace MR, Andersen LB, Saulino AM, Gregory PE, Glover TW, Collins FS. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991. 353: 864-6 117. Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM. Type 1 neurofibromatosis gene: Identification of a large transcript disrupted in three NF1 patients. Science. 1990. 249: 181-6 118. Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. von Recklinghausen’s disease and pheochromocytomas. J Urol. 1999. 162: 1582-6 119. Waltman L. A review of the literature on citation impact indicators. J Informetr. 2016. 10: 365-91 120. Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995. 146: 827-32 121. Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis Type 1 revisited. Pediatrics. 2009. 123: 124-33 122. Xu GF, Lin B, Tanaka K, Dunn D, Wood D, Gesteland R. The catalytic domain of the neurofibromatosis Type 1 gene product stimulates ras GTPase and complements ira mutants of S cerevisiae. Cell. 1990. 63: 835-41 123. Xu GF, O’Connell P, Viskochil D, Cawthon R, Robertson M, Culver M. The neurofibromatosis Type 1 gene encodes a protein related to GAP. Cell. 1990. 62: 599-608 124. Zöller ME, Rembeck B, Odén A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis Type 1 in a defined Swedish population. Cancer. 1997. 79: 2125-31 125. Zyoud SH, Fuchs-Hanusch D. A bibliometric-based survey on AHP and TOPSIS techniques. Expert systems with applications. 2017. 78: 158-81 126. Zyoud SH, Fuchs-Hanusch D. Mapping of climate change research in the Arab world: A bibliometric analysis. Environ Sci Pollut Res Int. 2020. 27: 3523-40 127. Zyoud SH, Zyoud AH. Coronavirus disease-19 in environmental fields: A bibliometric and visualization mapping analysis. Environ Dev Sustain. 2021. 23: 8895-923 128. Zyoud SH, Zyoud AH. Mapping environmental impact assessment research landscapes in the Arab world using visualization and bibliometric techniques. Environ Sci Pollut Res Int. 2021. 28: 22179-202 129. Zyoud SH, Zyoud SH, Al-Jabi SW, Sweileh WM, Awang R. Contribution of Arab countries to pharmaceutical wastewater literature: A bibliometric and comparative analysis of research output. Ann Occup Environ Med. 2016. 28: 28