- Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
Correspondence Address:
Mauro Dobran, Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
DOI:10.25259/SNI_276_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mauro Dobran, Serena Vittoria Lisi, Alessandro Di Rienzo, Erika Carrassi, Mara Capece, Pasquale Dorato, Lucia Giovanna Maria di Somma, Maurizio Iacoangeli. Evaluation of prognostic preoperative factors in patients undergoing surgery for spinal metastases: Results in a consecutive series of 81 cases. 19-Aug-2022;13:363
How to cite this URL: Mauro Dobran, Serena Vittoria Lisi, Alessandro Di Rienzo, Erika Carrassi, Mara Capece, Pasquale Dorato, Lucia Giovanna Maria di Somma, Maurizio Iacoangeli. Evaluation of prognostic preoperative factors in patients undergoing surgery for spinal metastases: Results in a consecutive series of 81 cases. 19-Aug-2022;13:363. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11815
Abstract
Background: Surgical treatment of spinal metastases should be tailored to provide pain control, neurological deficit improvement, and vertebral stability with low operative morbidity and mortality. The aim of this study was to analyze the predictive value of some preoperative factors on overall survival in patients undergoing surgery for spinal metastases.
Methods: We retrospectively analyzed a consecutive series of 81 patients who underwent surgery for spinal metastases from 2015 and 2021 in the Clinic of Neurosurgery of Ancona (Italy). Data regarding patients’ baseline characteristics, preoperative Karnofsky Performance Status Score (KPS), and Frankel classification grading system, histology of primary tumor, Tokuhashi revised and Tomita scores, Spine Instability Neoplastic Score, and Epidural Spinal Cord Compression Classification were collected. We also evaluated the interval time between the diagnosis of the primary tumor and the onset of spinal metastasis, the type of surgery, the administration of adjuvant therapy, postoperative pain and Frankel grade, and complications after surgery. The relationship between patients’ overall survival and predictive preoperative factors was analyzed by the Kaplan–Meier method. For the univariate and multivariate analysis, the log-rank test and Cox regression model were used. P ≤ 0.05 was considered as statistically significant.
Results: After surgery, the median survival time was 13 months. In our series, the histology of the primary tumor (P P P P P P P P P
Conclusion: These data suggest that patients with limited extension of primitive tumor and responsive to the adjuvant therapy are the best candidates for surgery with better outcome.
Keywords: Neurosurgery, Overall survival, Spinal cord compression, Spinal metastases, Spinal tumors
INTRODUCTION
Nowadays, advances in cancer treatment have prolonged patients’ life expectancy. Spinal metastases are a common occurrence in cancer patients. The most common sites for metastases in cancer patients are the liver and the lungs, followed by the spine.[
Breast, prostate, and lung cancer are the most frequent histologic type for spinal metastases. Spinal metastases are casually the first manifestation of an unknown primary tumor in about 10% of the patients.[
Since the therapy for primary tumors is continuously improving, the treatment of metastases is becoming one of the major challenges to prevent cancer-related disability and death. Surgical treatment should be chosen to provide the maximum palliative effect with a minimum operative morbidity and mortality for each patient.[
The purpose of the present study was to evaluate which preoperative factors predicted survival in patients with spinal metastasis undergoing surgery.
MATERIALS AND METHODS
We retrospectively analyzed the outcomes of a consecutive series of 81 patients who underwent surgical treatment for spinal metastases between January 2015 and January 2021 at the Clinic of Neurosurgery-Ancona (Italy). The follow-up ranged from 6 to 72 months.
Candidates for surgery were selected on the basis of three factors: (1) more than 6 months of life expectancy; (2) untreatable severe pain and/or presence of neurological deficits; and (3) need to collect tissue for diagnosis. Patients with previous surgery, aged <18 years old, and patients with diagnosis of multiple myeloma or lymphoma were excluded from this study.
All patients were preoperatively evaluated with computed tomography (CT) scan and magnetic resonance (MR) imaging of the spine. Chest, abdomen, and brain CT scan were also performed to detect systemic metastases. For each patient, we evaluated the following data: (1) demographics, (2) histology of primary tumor and systemic disease burden according to Tokuhashi revised and Tomita scores,[
The statistical analysis was performed by the software package SPSS, version 25.0 (Chicago, IL). We analyzed the relationship between patients’ overall survival and preoperative factors by the Kaplan–Meier method. For the univariate and multivariate analysis, the log-rank test and Cox regression model were used. P ≤ 0.05 was considered as statistically significant.
RESULTS
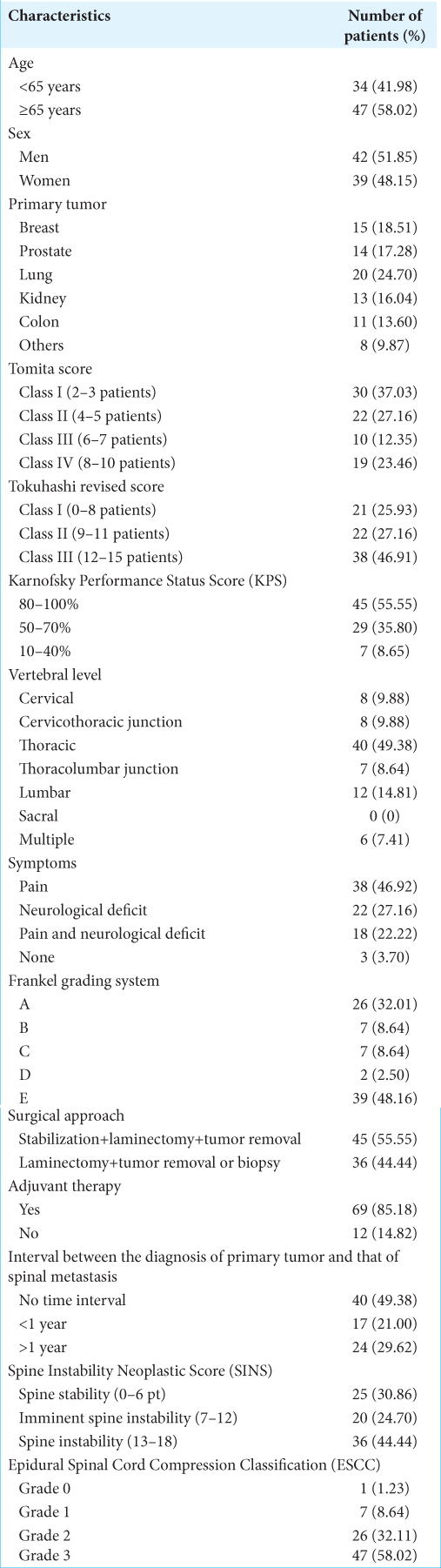
The outcomes of 81 consecutive patients (42 men and 39 women) with a median age of 67.0 ± 10.7 years (68.0 ± 8.2 in men and 65.0 ± 12.7 in women) were retrospectively reviewed. The preoperative characteristics of the study population are summarized in
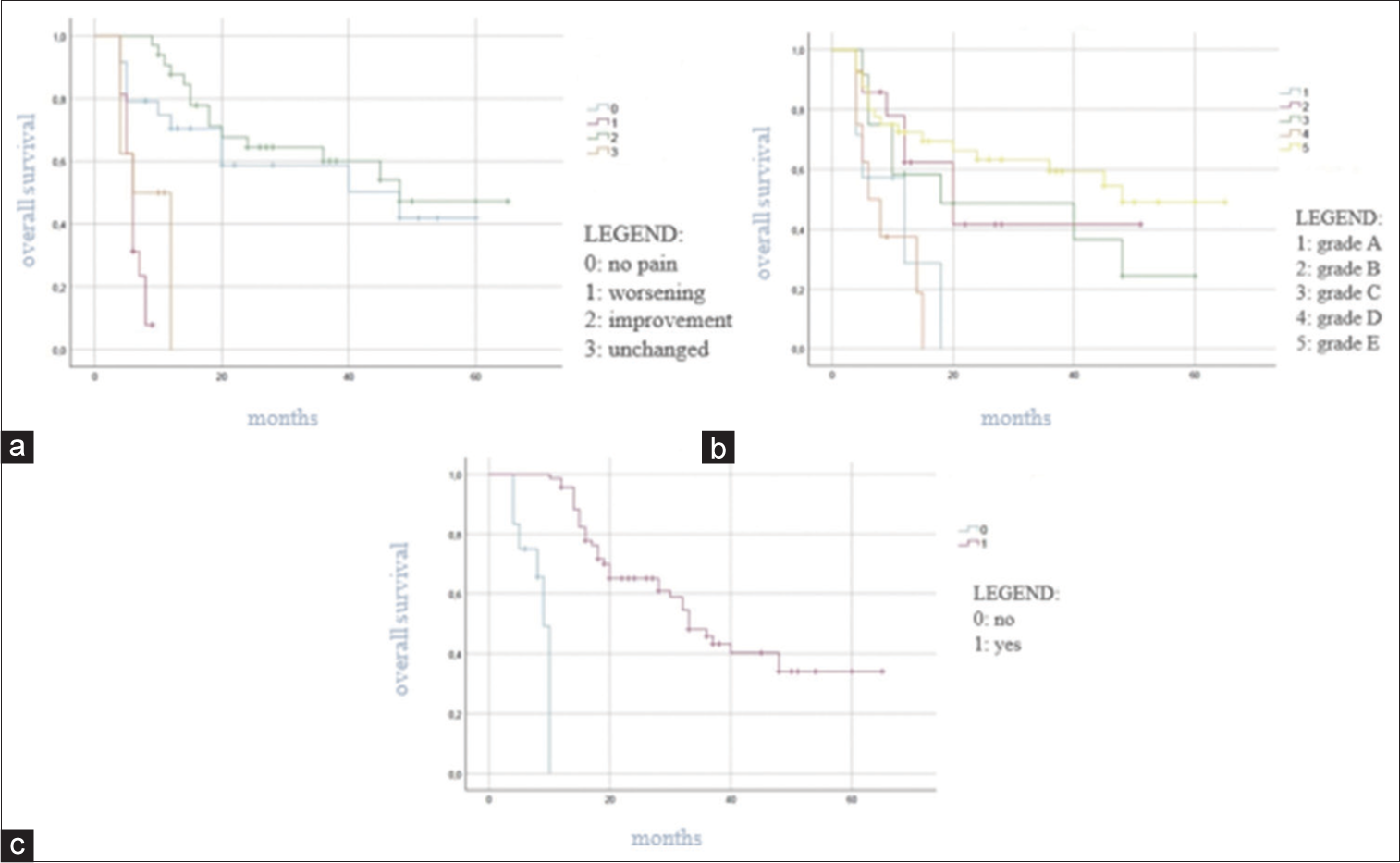
Breast, prostate, and lung cancers were the most common types of primary tumors. At admission, 78 patients out of 81 (96.3%) had pain and/or neurological deficits; in 26 patients out of 81 (32.01%), we documented a complete loss of motor and sensory function (Frankel Grade A). Most frequently spinal metastases were detected at the thoracic level (49.38%). According to the KPS score, patients were assigned to the following three groups: (1) scores 80–100% (45, 55.55%); (2) scores 50–70% (29, 35.8%); and (3) scores 10–40% (7, 8.65%). About 46.91% of all patients were included in Class III of Tokuhashi revised score; while 64.19% had been classified into Classes I and II of Tomita score [
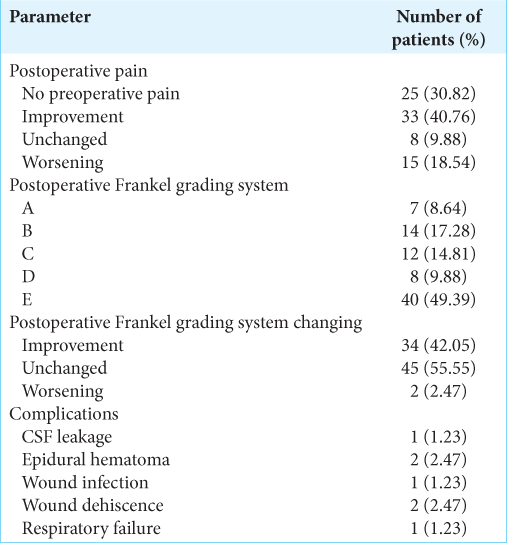
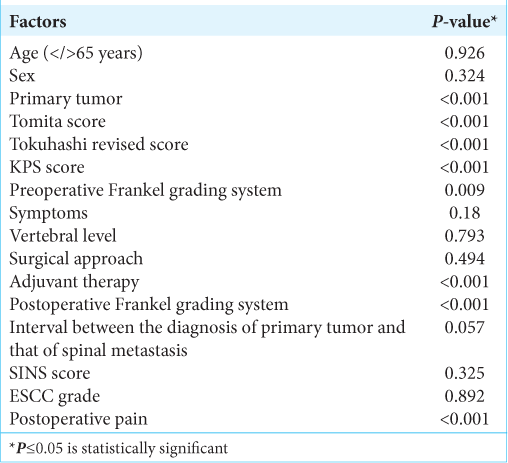
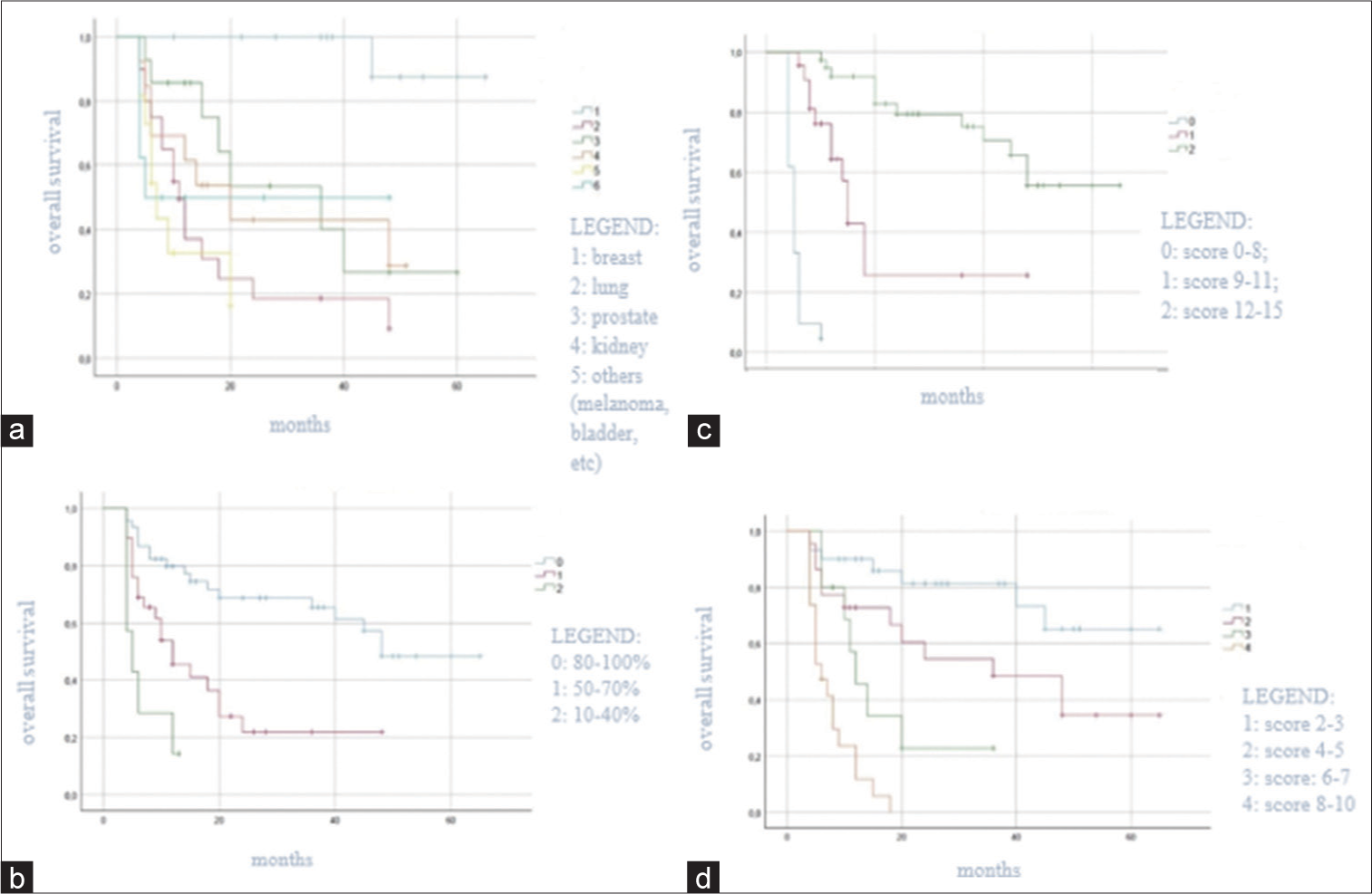
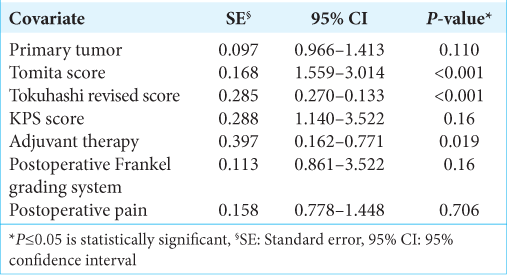
At univariate survival analysis, the histology of the primary tumor (<0.001), the preoperative KPS score (P < 0.001), the Tomita score (P < 0.001) and Tokuhashi revised score (P < 0.001), the administration of the adjuvant therapy (P < 0.001), the postoperative Frankel grade (P < 0.001), and the postoperative pain improvement (P < 0.001) were significantly related to overall survival [
DISCUSSION
The best treatment of spinal metastases requires an integrated approach with input from a multidisciplinary team comprised medical and radiation oncologists and neurosurgeons. In this regard, a new decision framework NOMS has been developed to tailor the treatment to each patient.[
In this study, we examined several preoperative factors to identify their prognostic value in patients operated for spinal metastases with the traditional decompression and maximal cytoreduction of the metastatic mass. Recently, a new technique of less aggressive surgery in vertebral metastases is performed by many authors with the name of separation surgery. This technique achieves the circumferential separation of the spinal cord from the tumor mass entrusting the control of the tumor growth to adjuvant therapy.[
Neither the epidural spinal cord compression based on ESCC scale nor the spinal instability defined by SINS score was statistically correlated with the overall survival in this study. However, the ESCC scale may play a role in early and proper diagnosis of spinal metastases and the SINS score may predict pathological fractures and radiotherapy failure.[
The limits of this study are the retrospective nature design and the single-center cohort with a relatively small population sample size.
CONCLUSION
The Tomita score, the Tokuhashi revised score, and the adjuvant therapy were statistically significant predictive factors for overall survival. The treatment of spinal metastases should be multifactorial and multidisciplinary, to obtain less operative morbidity and mortality with the maximum effectiveness.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aoude A, Amiot LP. A comparison of the modified Tokuhashi and Tomita scores in determining prognosis for patients afflicted with spinal metastasis. Can J Surg. 2014. 57: 188-93
2. Arrigo RT, Kalanithi P, Cheng I, Alamin T, Carragee EJ, Mindea SA. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011. 68: 674-81
3. Bilsky MH, Laufer I, Fourney DR, Groff M, Schmidt MH, Varga PP. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010. 13: 324-8
4. Campos M, Urrutia J, Zamora T, Román J, Canessa V, Borghero Y. The Spine instability neoplastic score: An independent reliability and reproducibility analysis. Spine J. 2014. 14: 1466-9
5. Cassidy JT, Baker JF, Lenehan B. The role of prognostic scoring systems in assessing surgical candidacy for patients with vertebral metastasis: A narrative review. Glob Spine J. 2018. 8: 638-51
6. Chen YJ, Chen HT, Hsu HC. Preoperative palsy score has no significant association with survival in non-small-cell lung cancer patients with spinal metastases who undergo spinal surgery. J Orthop Surg Res. 2015. 10: 149
7. De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017. 8: 25691-9
8. Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int. 2011. 108: 71-9
9. Di Perna G, Cofano F, Mantovani C, Badellino S, Marengo N, Ajello M. Separation surgery for metastatic epidural spinal cord compression: A qualitative review. J Bone Oncol. 2020. 25: 100320
10. Dobran M, Mancini F, Nasi D, Scerrati M. A case of deep infection after instrumentation in dorsal spinal surgery: The management with antibiotics and negative wound pressure without removal of fixation. BMJ Case Rep. 2017. p. 2017r-220792
11. Fourney DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011. 29: 3072-7
12. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH. The value of postural reduction in the initial management of closed injures of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969. 7: 179-92
13. Guzik G. Analysis of factors delaying the surgical treatment of patients with neurological deficits in the course of spinal metastatic disease. BMC Palliat Care. 2018. 17: 44
14. Hessler C, Vettorazzi E, Madert J, Bokemeyer C, Panse J. Actual and predicted survival time of patients with spinal metastases of lung cancer: Evaluation of the robustness of the Tokuhashi score. Spine (Phila Pa 1976). 2011. 36: 983-9
15. Huisman M, van der Velden JM, van Vulpen M, van den Bosch MA, Chow E, Öner FC. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J. 2014. 14: 2835-40
16. Karnofsky DA. Clinical evaluation of anticancer drugs. GANN Monogr. 1967. 2: 223-31
17. Kim J, Lee SH, Park SJ, Chung SS, Kim ES, Eoh W. Analysis of the predictive role and new proposal for surgical strategies based on the modified Tomita and Tokuhashi scoring systems for spinal metastasis. World J Surg Oncol. 2014. 12: 245
18. Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist. 2013. 18: 744-51
19. Lee BH, Kim TH, Chong HS, Moon ES, Park JO, Kim HS. Prognostic factor analysis in patients with metastatic spine disease depending on surgery and conservative treatment: Review of 577 cases. Ann Surg Oncol. 2013. 20: 40-6
20. Moon KY, Chung CK, Jahng TA, Kim HJ, Kim CH. Postoperative survival and ambulatory outcome in metastatic spinal tumors: Prognostic factor analysis. J Korean Neurosurg Soc. 2011. 50: 216-23
21. Onken JS, Fekonja LS, Wehowsky R, Hubertus V, Vajkoczy P. Metastatic dissemination patterns of different primary tumors to the spine and other bones. Clin Exp Metastasis. 2019. 36: 493-8
22. Padalkar P, Tow B. Predictors of survival in surgically treated patients of spinal metastasis. Indian J Orthop. 2011. 45: 307-13
23. Schick U, Marquardt G, Lorenz R. Intradural and extradural spinal metastases. Neurosurg Rev. 2001. 24: 1-5
24. Tabouret E, Gravis G, Cauvin C, Loundou A, Adetchessi T, Fuentes S. Long-term survivors after surgical management of metastatic spinal cord compression. Eur Spine J. 2015. 24: 209-15
25. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005. 30: 2186-91
26. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001. 26: 298-306
27. Tseng CL, Eppinga W, Charest-Morin R, Soliman H, Myrehaug S, Maralani PJ. Spine stereotactic body radiotherapy: Indications, outcomes and points of caution. Glob Spine J. 2017. 7: 179-97
28. Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003. 28: 508-12
29. Yahanda AT, Buchowski JM, Wegner AM. Treatment, complications, and outcomes of metastatic disease of the spine: From patchell to PROMIS. Ann Transl Med. 2019. 7: 216
30. Yang SB, Cho W, Chang UK. Analysis of prognostic factors relating to postoperative survival in spinal metastases. J Korean Neurosurg Soc. 2012. 51: 127-34