- Department of Neurosurgery, Hamamatsu University School of Medicine,
- Department of Neurosurgery, Hamamatsu Medical Center, Hamamatsu, Japan.
Correspondence Address:
Hiroaki Neki, Department of Neurosurgery, Hamamatsu University School of Medicine, Hamamatsu, Japan.
DOI:10.25259/SNI_625_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Satoru Kida1, Hiroaki Neki1, Hisaya Hiramatsu2, Yoshinobu Kamio1, Ippei Makita1, Yuki Shiraishi1, Kazuhiko Kurozumi1. De novo dural arteriovenous fistula after mechanical thrombectomy for cerebral venous thrombosis: A case report. 09-Sep-2022;13:411
How to cite this URL: Satoru Kida1, Hiroaki Neki1, Hisaya Hiramatsu2, Yoshinobu Kamio1, Ippei Makita1, Yuki Shiraishi1, Kazuhiko Kurozumi1. De novo dural arteriovenous fistula after mechanical thrombectomy for cerebral venous thrombosis: A case report. 09-Sep-2022;13:411. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11855
Abstract
Background: Although the relationship between dural arteriovenous fistula (dAVF) and cerebral venous thrombosis (CVT) has been reported, the etiology has not been clarified. Here, we report a case of de novo dAVF after mechanical thrombectomy for CVT and discuss the underlying mechanism.
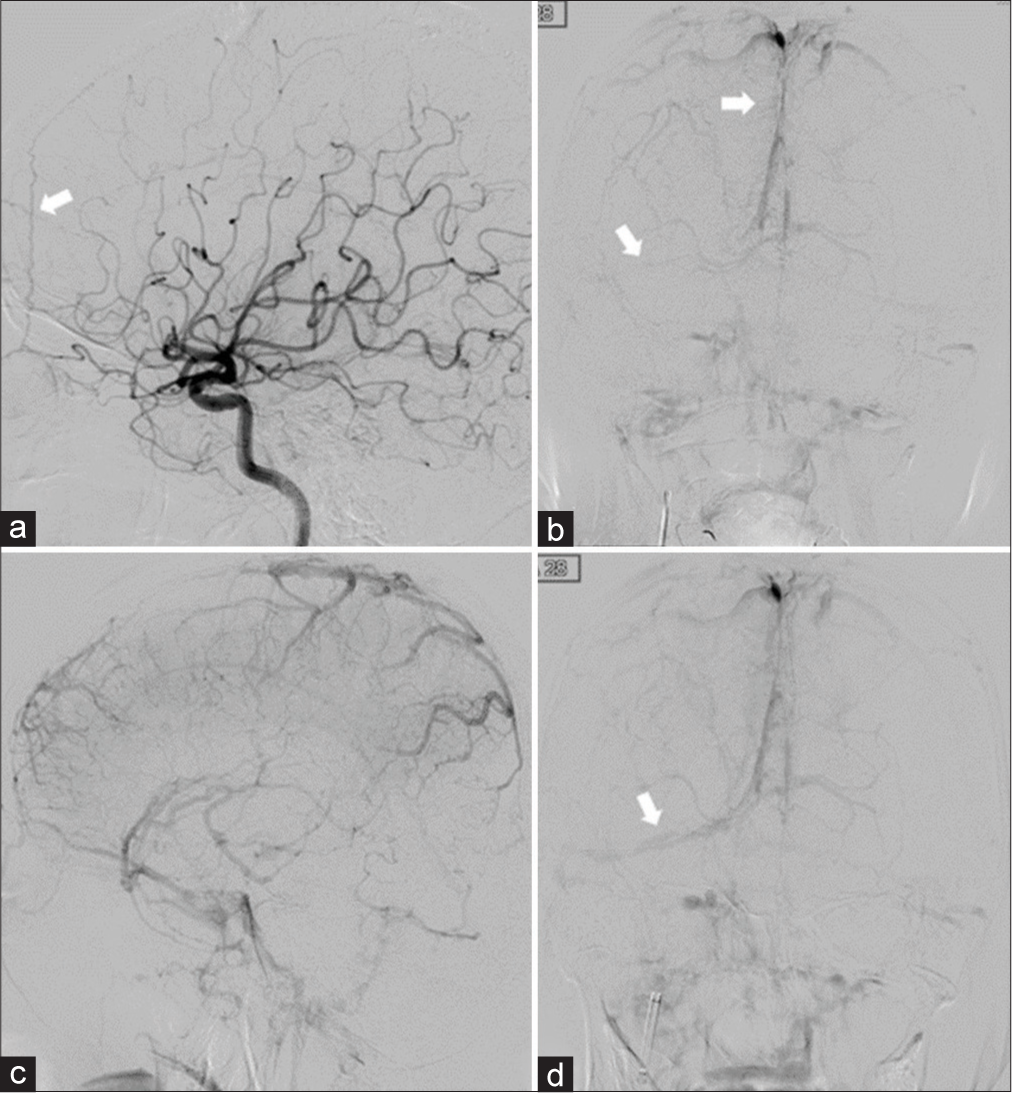
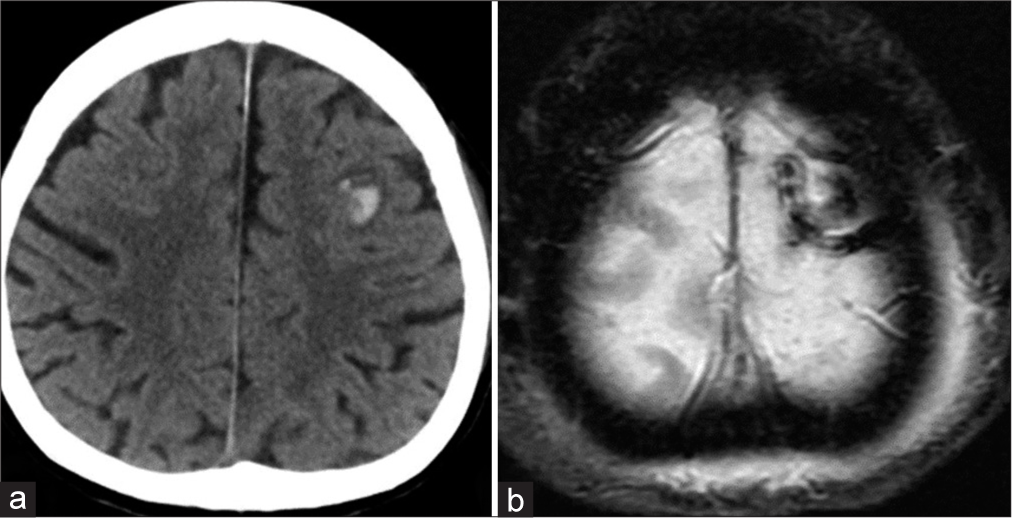
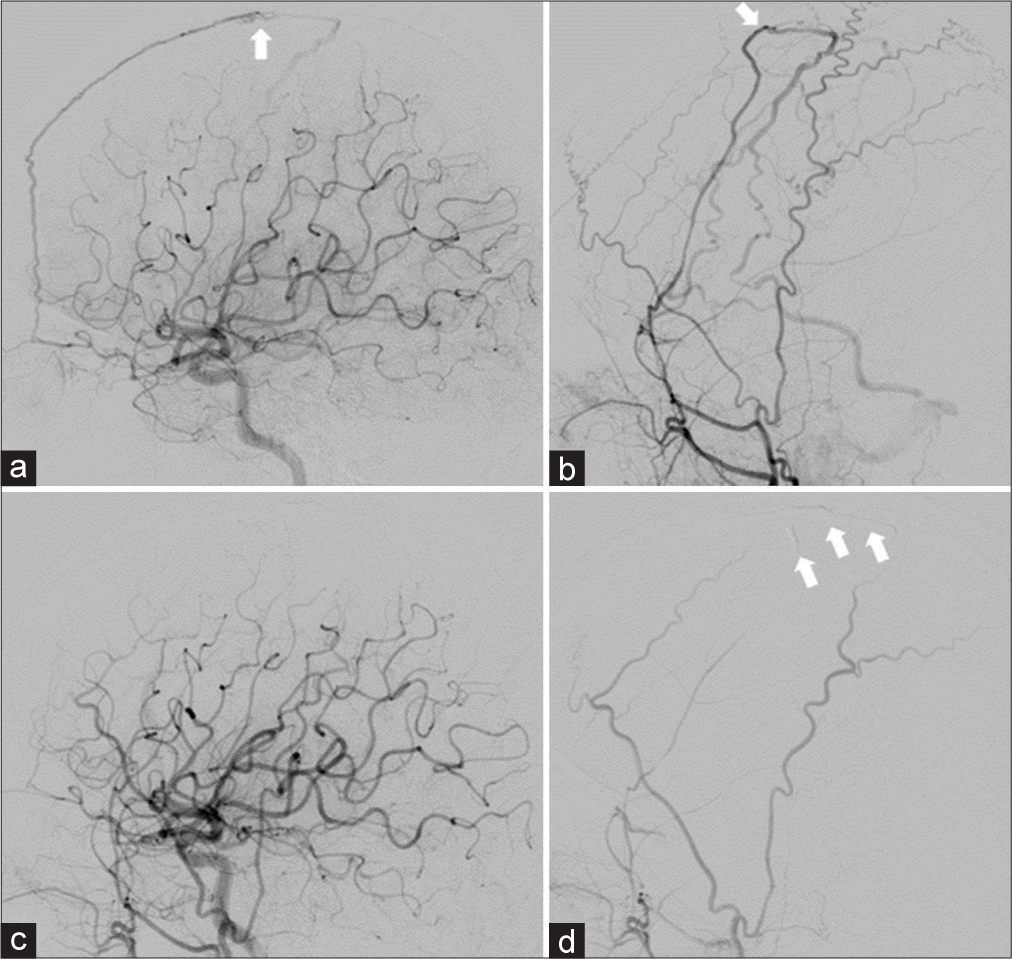
Case Description: A 61-year-old woman presented with a gradually worsening headache and was diagnosed with severe CVT. Mechanical thrombectomy was performed for the CVT because of progressive neurological deterioration despite anticoagulation therapy. Two years after the initial treatment, angiography revealed a de novo dAVF with a direct shunt of the left convexity cortical vein. Transarterial embolization with Onyx was performed and the shunt was completely obliterated.
Conclusion: In this report, we describe a case of de novo dAVF with CVT that was treated with mechanical thrombectomy. Even if CVT improves with mechanical thrombectomy, we must be aware of the occurrence of de novo dAVF.
Keywords: Cerebral venous thrombosis, Dural arteriovenous fistula, Mechanical thrombectomy
INTRODUCTION
Dural arteriovenous fistulas (dAVF) are acquired following cerebral venous thrombosis (CVT), trauma, or surgery.[
CASE DESCRIPTION
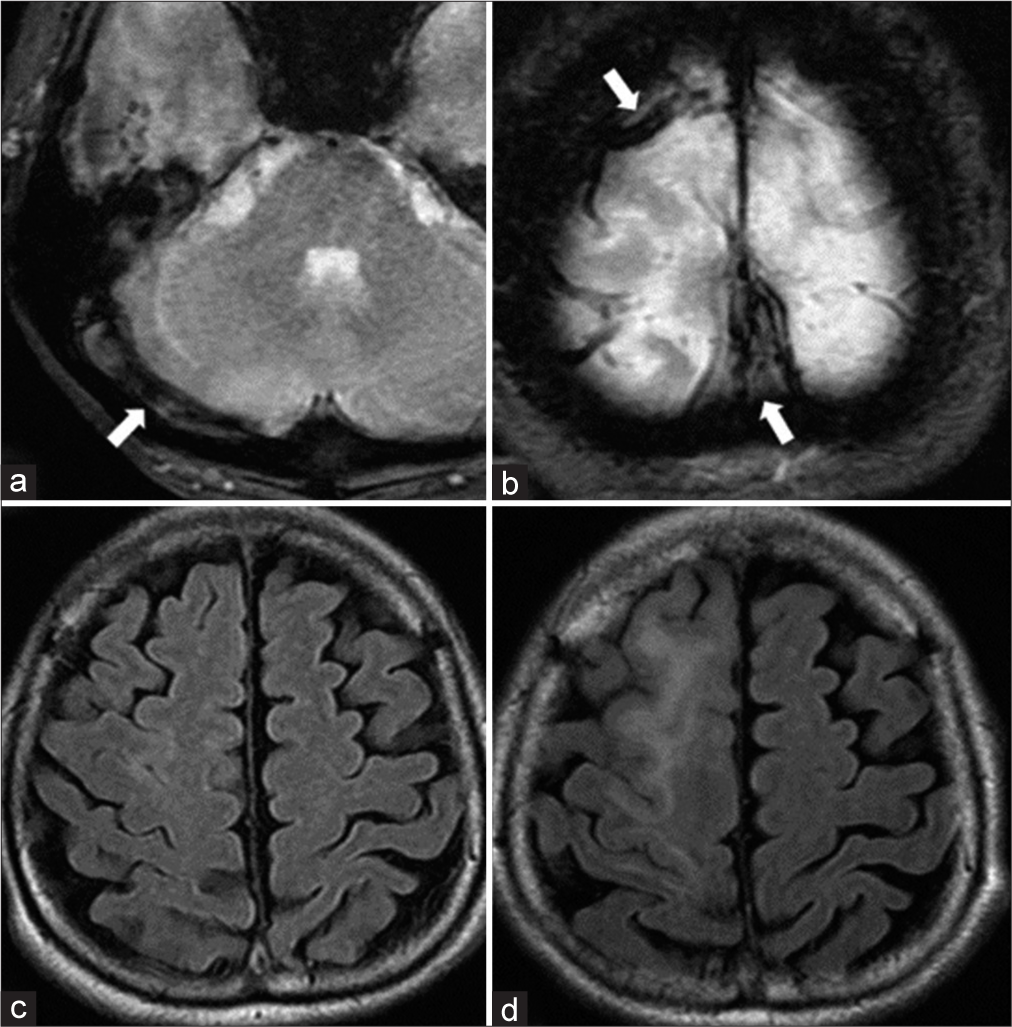
A 61-year-old woman presented with a history of idiopathic thrombocytopenic purpura. She presented with gradually worsening headache, visual disturbance, and disorientation for approximately 3 weeks. Neurological examination revealed confused consciousness and headache worsening in the recumbent position. Diffusion-weighted images demonstrated multiple infarctions in the right temporal and cerebellar hemispheres. T2*-weighted images revealed an extensive thrombus affecting the superior sagittal sinus (SSS) to the right sigmoid sinus (SS) [
DISCUSSION
The previous studies have reported that dAVFs are acquired due to CVT, trauma, surgery, coagulopathy, and infection.[
In a previous study investigating the characteristics and risk factors of dAVF with CVT in a large multicenter cohort of patients with CVT, dAVF patients with CVT had multiple dAVFs in 50% of them, and SS and TS were involved in approximately 70% each. Regarding the Cognard type, IIa+b was the most common, followed by IIa and III. Compared to CVT-only patients, they were older (median age 53 years), more often male, had a more chronic CVT course (>30 days), and more often had thrombosis in the SS, but with fewer parenchymal brain lesions.[
The mechanism of acquired dAVF after CVT is generally suspected to be caused by increased venous pressure due to CVT and not by the venous thrombus itself. Venous hypertension due to steno-occlusive lesions in the venous sinus dilates the venules and can result in arteriovenous shunt formation.[
The first cause of de novo dAVF is insufficient venous pressure reduction. Due to partial recanalization of the dural sinuses, venous pressure decreased to some extent. However, venous congestion persisted despite mechanical thrombectomy due to the high thrombus volume. Consequently, the remaining venous hypertension persisted, resulting in dAVF formation. Moreover, the fact that the anterior falcine artery was slightly prominent on the first angiography could be a sign that de novo dAVF formation had already begun to develop due to initial venous hypertension. Second, cortical vein rethrombosis could be involved in dAVF formation. Although the overall venous pressure was decreased by mechanical thrombectomy, local venous hypertension could occur due to partial venous stagnation induced by thrombus in the cortical vein, which caused the dAVF. Regarding the nonsupportive point for this hypothesis, there was no dAVF resulting from cortical vein thrombosis in the aforementioned study.[
In summary, we encountered a rare case of de novo dAVF after mechanical thrombectomy for severe CVT. Although neurological disorders caused by CVT were improved by mechanical thrombectomy, multiple factors were present that could cause de novo dAVF, such as insufficient venous pressure reduction, cortical venous re-thrombosis, and large-bore catheter treatment. In this case, early diagnosis and intervention could have prevented intracranial hemorrhage and nonhemorrhagic aggressive symptoms due to dAVF. We must be aware that even if venous pressure reduction appears to be achieved for CVT patients, high-grade dAVF could still occur following CVT. Therefore, careful follow-up is warranted.
CONCLUSION
We present a rare case of de novo dAVF after mechanical thrombectomy for severe CVT. Even if CVT improves by mechanical thrombectomy, there is a need to keep in mind the occurrence of de novo dAVF.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baharvahdat H, Ooi YC, Kim WJ, Mowla A, Coon AL, Colby GP. Updates in the management of cranial dural arteriovenous fistula. Stroke Vasc Neurol. 2020. 5: 50-8
2. Chen L, Mao Y, Zhou LF. Local chronic hypoperfusion secondary to sinus high pressure seems to be mainly responsible for the formation of intracranial dural arteriovenous fistula. Neurosurgery. 2009. 64: 973-83
3. Demartini Z, Gatto LA, de Oliveira TV, Guimaraes RM, Francisco AN, Koppe GL. Dural arteriovenous fistula associated with dental implant. World Neurosurg. 2020. 141: 69-71
4. Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992. 89: 465-73
5. Hamada Y, Goto K, Inoue T, Iwaki T, Matsuno H, Suzuki S. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997. 40: 452-56
6. Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995. 83: 539-45
7. Houser OW, Campbell JK, Campbell RJ, Sundt TM. Arteriovenous malformation affecting the transverse dural venous sinus--an acquired lesion. Mayo Clin Proc. 1979. 54: 651-61
8. Lasjaunias P, Magufis G, Goulao A, Piske R, Suthipongchai S, Rodesch R. Anatomoclinical aspects of dural arteriovenous shunts in children. Review of 29 cases. Interv Neuroradiol. 1996. 2: 179-91
9. Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997. 87: 267-74
10. Lindgren E, Rentzos A, Hiltunen S, Serrano F, Heldner MR, Zuurbier SM. Dural arteriovenous fistulas in cerebral venous thrombosis: Data from the international cerebral venous thrombosis consortium: Data from the international cerebral venous thrombosis consortium. Eur J Neurol. 2022. 29: 761-70
11. Mironov A. Pathogenetical consideration of spontaneous dural arteriovenous fistulas (DAVFs). Acta Neurochir (Wien). 1994. 131: 45-58
12. Piippo A, Niemelä M, van Popta J, Kangasniemi M, Rinne J, Jääskeläinen JE. Characteristics and long-term outcome of 251 patients with dural arteriovenous fistulas in a defined population. J Neurosurg. 2013. 118: 923-34
13. Shin Y, Nakase H, Nakamura M, Shimada K, Konishi N, Sakaki T. Expression of angiogenic growth factor in the rat DAVF model. Neurol Res. 2007. 29: 727-33
14. Terada T, Tsuura M, Komai N, Higashida RT, Halbach VV, Dowd CF. The role of angiogenic factor bFGF in the development of dural AVFs. Acta neurochir (Wien). 1996. 138: 877-83
15. Van Dijk JM, TerBrugge KG, Van der Meer FJ, Wallace MC, Rosendaal FR. Thrombophilic factors and the formation of dural arteriovenous fistulas. J Neurosurg. 2007. 107: 56-9