- Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University,
- Department of Neurosurgery, Kagoshima City Hospital, Kagoshima, Japan,
- Department of Neonatology, Kagoshima City Hospital, Kagoshima, Japan.
Correspondence Address:
Masanori Sato, Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
DOI:10.25259/SNI_629_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masanori Sato1, Tatsuki Oyoshi2, Hirofumi Iwamoto1, Natsuko Tanoue1, Soichiro Komasaku1, Nayuta Higa1, Hiroshi Hosoyama1, Hiroshi Tokimura2, Satoshi Ibara3, Ryosuke Hanaya1, Koji Yoshimoto1. The collagen matrix dural substitute graft prevents postoperative cerebrospinal fluid leakage after ventriculoperitoneal shunt surgery in patients aged
How to cite this URL: Masanori Sato1, Tatsuki Oyoshi2, Hirofumi Iwamoto1, Natsuko Tanoue1, Soichiro Komasaku1, Nayuta Higa1, Hiroshi Hosoyama1, Hiroshi Tokimura2, Satoshi Ibara3, Ryosuke Hanaya1, Koji Yoshimoto1. The collagen matrix dural substitute graft prevents postoperative cerebrospinal fluid leakage after ventriculoperitoneal shunt surgery in patients aged https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11917
Abstract
Background: Cerebrospinal fluid (CSF) leakage is a common complication of ventriculoperitoneal shunt (VPS) and has the potential to induce shunt infection. Especially in infants and children, these are serious complications. DuraGen is a collagen matrix dural substitute used to reduce the risk of CSF leakage in various neurosurgeries. We report our VPS procedure with DuraGen for preventing postoperative CSF leakage in patients aged
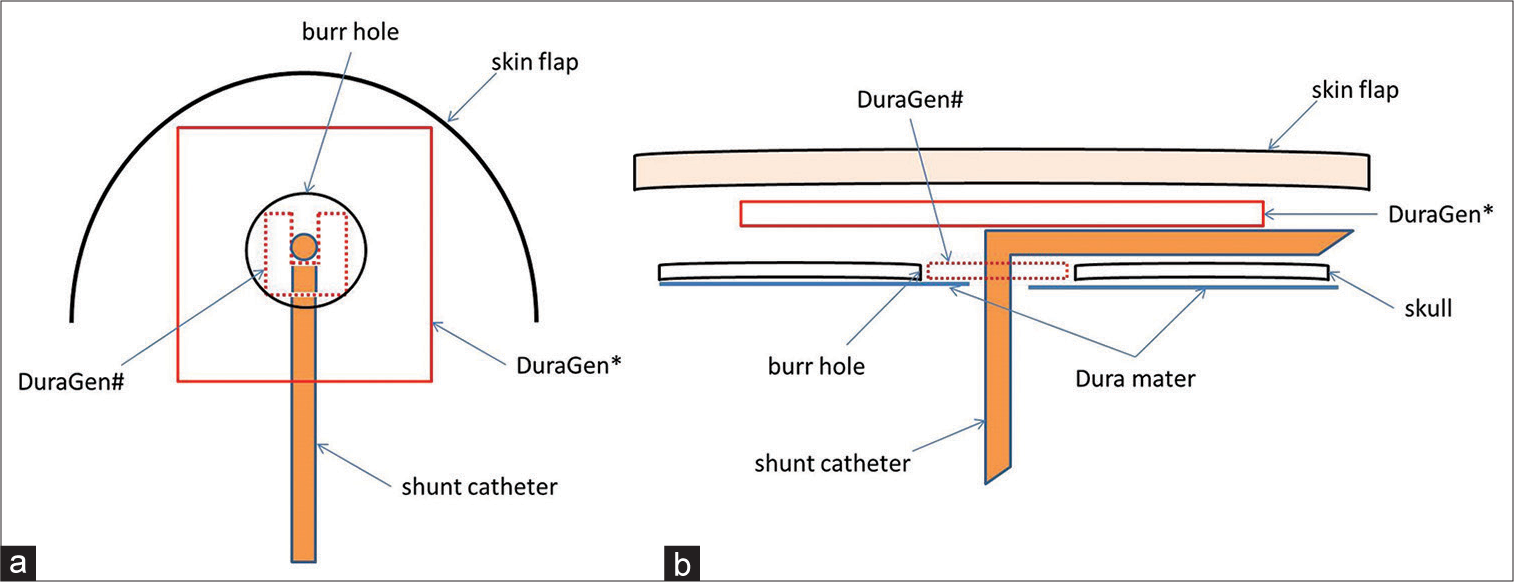
Methods: We used DuraGen to prevent postoperative CSF leakage in six VPS surgeries. Antibiotic-impregnated shunt catheters and programmable valves with anti-siphon devices were also used in all cases. DuraGen was placed inside and atop the burr hole. All cases had an initial shunt pressure of 5 cmH2O. Fibrin glue was not used.
Results: The patients underwent follow-up for a year after VPS surgery. There was no postoperative subcutaneous CSF collection or leakage after all six VPS surgeries. Furthermore, no postoperative shunt infections or DuraGen-induced adverse events were noted.
Conclusion: We speculate that DuraGen has a preventive effect on postoperative CSF leakage in VPS cases aged
Keywords: Cerebrospinal fluid leakage, Collagen matrix, Hydrocephalus, Pediatric neurosurgery, Shunt infection, Ventriculoperitoneal shunt
INTRODUCTION
A ventriculoperitoneal shunt (VPS) is a common procedure for hydrocephalus in newborns and infants. Cerebrospinal fluid (CSF) leakage is a common complication of VPS, occurring in about 7% of patients who have undergone VPS.[
Shunt infection is a serious complication that could lead to severe morbidity and mortality;[
The collagen matrix acts as a scaffold on which host fibroblasts lay down new collagen, which promotes fibrin clot formation, with full reabsorption with the dura healing and integration into the endogenous dura mater.[
Herein, we report our VPS procedure with DuraGen for preventing postoperative CSF leakage in patients aged <1 year.
MATERIALS AND METHODS
We used DuraGen in all VPS surgeries in patients aged <1 year at Kagoshima City Hospital between June 2020 and December 2020. We performed six VPS surgeries with DuraGen (including one revision VPS surgery) in this period. Nine patients aged <1 year who underwent VPS surgery with antibiotic-impregnated shunt catheters (AISCs): Bactiseal (Codman, Johnson and Johnson, Raynham, Massachusetts, USA) and programmable valves: proGAV 2.0 (Miethke, Aesculap, Potsdam, Tuttlingen, Germany) or Hakim Medos (Codman, Johnson and Johnson, Raynham, Massachusetts, USA) without DuraGen (including two revision VPS surgeries) before June 2020 were considered the control group (non-DuraGen).
RESULTS
All patients underwent follow-up for a year after VPS surgery. There was no consistent postoperative subcutaneous CSF collection or leakage (zero of six surgeries) in the DuraGen group; in contrast, six cases of CSF leakage (including subcutaneous CSF collection) were observed in the non-DuraGen group. There were also no cases and two cases of postoperative shunt infection in the DuraGen and non-DuraGen groups, respectively. Moreover, two cases of shunt infection with CSF leakage that required revision surgery were noted in the non-DuraGen group, while one case of ventral tube obstruction without infection that required revision surgery was reported in the DuraGen group. In addition, DuraGen-induced adverse events were not observed in all surgeries.
DISCUSSION
Our previous nine patients with VPS aged <1 year using AISCs and programmable valves without DuraGen had six subcutaneous CSF collections or CSF leakage and two infections. It was suggested that DuraGen has a preventive effect on CSF leakage in patients <1 year.
Collagen matrix is commonly used in dural closure and plasty in various neurosurgeries. Graft encapsulation, neo-membrane formation, delayed bleeding, and foreign body reaction have not been reported in dural plasty with collagen matrix dural substitutes.[
DuraGen is a sutureless dural substitute graft. Collagen matrix dural substitutes initially adhere to the dura through surface tension.[
Despite these findings, a limitation of this nonrandomized controlled study was its small sample size. DuraGen was used in our VPS procedure for the 1st time in June 2020 with the aim of preventing CSF leakage, as shown in
CONCLUSION
Our findings suggest that DuraGen can be safely used in neonates and infants, and it has a preventive effect on CSF leakage.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams DJ, Rajnik M. Microbiology and treatment of cerebrospinal fluid shunt infections in children. Curr Infect Dis Rep. 2014. 16: 427
2. Çetin B, Şengül G, Tüzün Y, Gündoğdu C, Kadıoğlu HH, Aydın İH. Suitability of collagen matrix as a dural graft in the repair of experimental posterior fossa dura mater defects. Turk Neurosurg. 2006. 16: 9-13
3. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004. 350: 1422-9
4. Davis SE, Levy ML, McComb JG, Masri-Lavine L. Does age or other factors influence the incidence of ventriculoperitoneal shunt infections?. Pediatr Neurosurg. 1999. 30: 253-7
5. Forward KR, Fewer HD, Stiver HG. Cerebrospinal fluid shunt infections. A review of 35 infections in 32 patients. J Neurosurg. 1983. 59: 389-94
6. Harrop JS, Sharan AD, Ratliff J, Prasad S, Jabbour P, Evans JJ. Impact of a standardized protocol and antibiotic-impregnated catheters on ventriculostomy infection rates in cerebrovascular patients. Neurosurgery. 2010. 67: 187-91 discussion 191
7. Jeelani NU, Kulkarni AV, Desilva P, Thompson DN, Hayward RD. Postoperative cerebrospinal fluid wound leakage as a predictor of shunt infection: A prospective analysis of 205 cases. Clinical article. J Neurosurg Pediatr. 2009. 4: 166-9
8. Knopp U, Christmann F, Reusche E, Sepehrnia A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects--a comparative animal experimental study. Acta Neurochir (Wien). 2005. 147: 877-87
9. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: A prospective study of risk factors. J Neurosurg. 2001. 94: 195-201
10. Lullove E. Acellular fetal bovine dermal matrix in the treatment of nonhealing wounds in patients with complex comorbidities. J Am Podiatr Med Assoc. 2012. 102: 233-9
11. McGirt MJ, Leveque JC, Wellons JC, Villavicencio AT, Hopkins JS, Fuchs HE. Cerebrospinal fluid shunt survival and etiology of failures: A seven-year institutional experience. Pediatr Neurosurg. 2002. 36: 248-55
12. Narotam PK, Van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995. 82: 406-12
13. Neulen A, Gutenberg A, Takács I, Wéber G, Wegmann J, Schulz-Schaeffer W. Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural on lay graft in a large animal model. Acta Neurochir (Wien). 2011. 153: 2241-50
14. Raffa G, Marseglia L, Gitto E, Germanò A. Antibiotic-impregnated catheters reduce ventriculoperitoneal shunt infection rate in high-risk newborns and infants. Childs Nerv Syst. 2015. 31: 1129-38
15. Rohde V, Weinzierl M, Mayfrank L, Gilsbach JM. Postshunt insertion CSF leaks in infants treated by an adjustable valve opening pressure reduction. Childs Nerv Syst. 2002. 18: 702-4
16. Rotim K, Miklic P, Paladino J, Melada A, Marcikic M, Scap M. Reducing the incidence of infection in pediatric cerebrospinal fluid shunt operations. Childs Nerv Syst. 1997. 13: 584-7
17. Shannon CN, Simon TD, Reed GT, Franklin FA, Kirby RS, Kilgore ML. The economic impact of ventriculoperitoneal shunt failure. J Neurosurg Pediatr. 2011. 8: 593-9
18. Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr. 2009. 4: 156-65
19. Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: Utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008. 1: 131-7
20. Wang KW, Chang WN, Shih TY, Huang CR, Tsai NW, Chang CS. Infection of cerebrospinal fluid shunts: Causative pathogens, clinical features, and outcomes. Jpn J Infect Dis. 2004. 57: 44-8