- Department of Vascular Neurosurgery, Instuto Nacional de Neurología y Neurocirugía “Manuel Velasco Suárez” - Mexico City, Mexico.
- Department of Neurological Surgery, New York-Presbyterian Hospital, Weill Cornell Medical College, New York, United States,
- Department of Neurological Surgery, Hospital Civil de Guadalajara. Guadalajara City, Mexico.
Correspondence Address:
Edgar Nathal, Department of Vascular Neurosurgery, Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suárez” - Mexico City.
DOI:10.25259/SNI_572_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Javier Degollado-García1, Mauricio Medina-Pizarro2, Gerardo Cano-Velazquez1, Juan C. Balcázar-Padrón1, Oscar Gutierrez-Avila3, Edgar Nathal1. Microsurgical treatment of carotid body tumors using periadventitial dissection: Analysis of outcomes and prognostic factors in a neurological referral center. 28-Oct-2022;13:487
How to cite this URL: Javier Degollado-García1, Mauricio Medina-Pizarro2, Gerardo Cano-Velazquez1, Juan C. Balcázar-Padrón1, Oscar Gutierrez-Avila3, Edgar Nathal1. Microsurgical treatment of carotid body tumors using periadventitial dissection: Analysis of outcomes and prognostic factors in a neurological referral center. 28-Oct-2022;13:487. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11969
Abstract
Background: Surgical resection for carotid body tumors (CBTs) is the gold standard of treatment and continues to be a challenging procedure, commonly associated with high vascular injury rates and neurological complications.
Methods: It is a retrospective case series study between January 2002 and November 2020, with a mean follow-up of 29 months in a single nationwide referral center. Thirty-one patients diagnosed with a carotid body tumor and treated with microsurgical periadventitial resection were included in the study. Patients’ demographics, comorbidities, clinical, radiological factors, and tumor grade, evaluated by the Shamblin scale, were obtained. Statistical analysis was performed on all collected data.
Results: In this study, we included 31 patients (32 tumors), 80% of the patients were female, and 20% were male, with a mean age of 53 years. One patient presented with bilateral lesions, while 17 tumors were located on the left side. The most frequent symptom was a painless, slow-growing neck mass in 74% of patients. Using the Shamblin classification, 13% of tumors were Grade I, 53% Grade II, and 34% Grade III. In the postoperative period, 3% of patients presented with permanent cranial nerve deficit, while none had vascular injuries or postoperative stroke. A tumor >5 cm increased the risk for nerve lesion by 11 times (OR 12.6, CI 95% 7.4-11.4, P
Conclusion: Preoperative embolization followed by periadventitial resection by means of a microsurgical technique is a safe and effective approach to remove CBT, with 3% cranial nerve injury rate and no need for vascular sacrifice or reconstruction.
Keywords: Carotid body tumor, Microsurgical treatment, Paraganglioma, Periadventitial dissection, Shamblin grade
INTRODUCTION
Carotid body tumors (CBTs) are included in the classification of paragangliomas. These tumors are rare, with an estimated incidence of 1:30,000–100,000 and only 3% appearing in the head and neck.[
The aim of this study is to review a single-institution experience in the surgical management of CBTs and evaluate the outcomes and complications using a periadventitial microsurgical technique preceded by preoperative embolization.
MATERIALS AND METHODS
Study design
A single-center and retrospective case series of patients with CBTs treated with the periadventitial microsurgical technique by the senior surgeon (EN) between January 2002 and November 2020 was performed. Preoperative, intraoperative, and postoperative data were collected from charts and analyzed for each patient. Tumors were classified according to the criteria established by Shamblin et al.[
Before the surgery, in all case, a detailed digital subtraction angiography (DSA) was performed through the femoral artery. Then, a computed tomography (CT) angiogram, magnetic resonance imaging (MRI), or magnetic resonance angiography were obtained. Tumor volume was estimated on the preoperative MRI by the following formula: 4/3 π*(abc) with a, b, and c being the tumor diameters in each of the three dimensions. According to postoperative imaging studies (CT scan and MRI), the resection degree was classified as complete or incomplete. Surgical bleeding was estimated by the anesthesiologist based on the count of gauze pads and containers and reported in each of the patient’s charts. Finally, recurrence was defined as tumor growth in any of its dimensions during follow-up and until the patient’s last recorded visit.
Statistical analysis
Descriptive analysis of data was made using mean, median, percentages, and maximum and minimum values. Continuous variables were represented by mean and standard deviation with range values, except in cases otherwise specified. Correlation analysis between variables was obtained employing calculating the Spearman coefficient for non-Gaussian distributed variables. Linear regression and a penalized likelihood method were used to calculate a multivariable logistic regression model to assess for predictors of surgical outcome, nerve injury, and severe blood loss. Data were analyzed and processed with SPSS version 21 from IBM, Stata 2021 (Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC), and Microsoft Excel. Statistical significance was taken as P < 0.05. In linear and logistic regression models, significance was taken with P < 0.05 and when confidence intervals did not include 1.
Microsurgical technique
Patients are positioned in dorsal decubitus with a rolled sheet underneath the shoulders, with the head resting on a horseshoe head holder with the neck hyperextended and the head rotated 20° toward the contralateral side of the lesion. Asepsis of the chin, mastoid, and cervical region is performed on the lesion side, and the placement of sterile dressing is performed as usual. An incision is made on the anterior edge of the sternocleidomastoid muscle, from the mandibular angle to the upper edge of the thyroid cartilage; the superficial fascia and the first layer of the deep fascia are opened to continue mobilizing the sternocleidomastoid muscle laterally approaching the carotid triangle (digastric muscle at the base of the triangle, the sternocleidomastoid laterally, and the omohyoid muscle medially). The sheath is incised to dissect the common carotid from the internal jugular, the vagus, hypoglossal, and superficial laryngeal nerve (when visible), up to the level of the carotid bifurcation, where the tumor is identified in its whole extension. Vessel loops are placed on the common carotid to achieve proximal control if necessary. Under magnification with a surgical microscope (OPMI PENTERO 900 Carl Zeiss AG, Oberkochen, Germany), the tumor is dissected from the adventitia by recognizing a white line between them. This is the most important step when treating tumors Shambling II or III because the carotid is not easily visible and can be damaged. To get this initial dissection, we use a combination of blunt dissection, low-power bipolar coagulation, and micro-scissor cut, preserving the nerves until the lesion is progressively isolated from the external and internal carotid sides. In all Shamblin III tumors, we use intraoperative Doppler assistance in this step to identify the carotid wall. Once the carotid arteries are separated from the tumor, vascular loops are used to mobilize them when the tumor is being dissected. Next, the base of the tumor at the carotid bifurcation is approached in the same way, using the bipolar forceps in a mid-power energy range (12–15 units), avoiding heat damage to the artery wall. In this sense, the preoperative embolization and the surgical microscope are extremely helpful in maintaining the blood loss at a very low level. Finally, in most cases, a fibrous band present at the apical part of the tumor is cut to liberate the lesion completely. Following this, adequate hemostasis of the field is granted, and closure is performed in a multilayered fashion, facing the superficial fascia and subcutaneous tissue with 3–0 Vicryl with a continuous suture and the skin with 3–0 nylon subdermal stitch. Images of three representative cases are shown in
Figure 1:
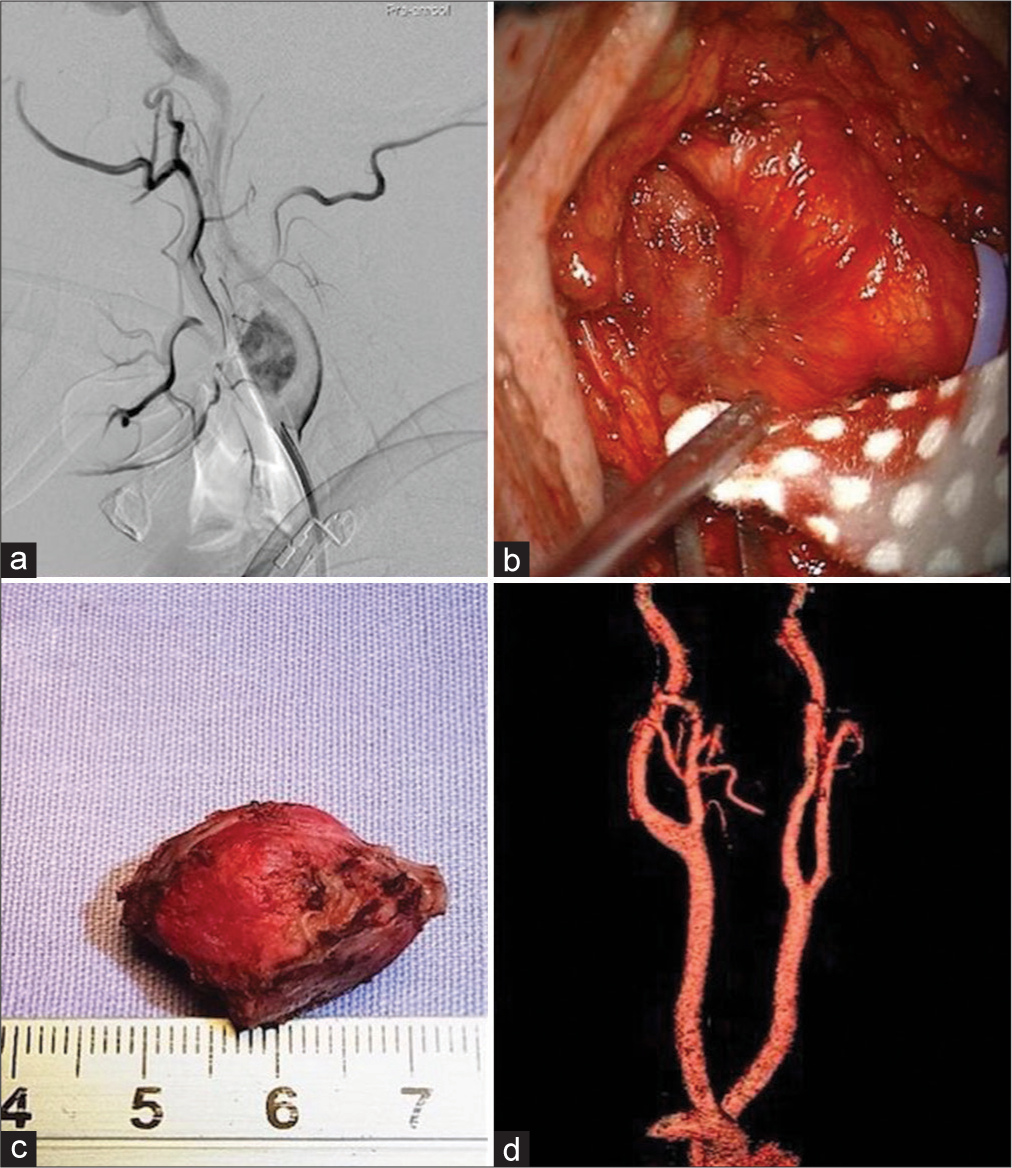
Case 1. Right side carotid body tumor (CBT), Shamblin I. (a) Preoperative DSA showing the vascularity of the tumor. (b) Surgical exposure of the carotid bifurcation. The tumor is visible between the external and internal carotid arteries. (c) Tumor after extraction from the surgical bed. (d) Postoperative computed tomography (CT) angio showing complete resection of the tumor.
Figure 2:
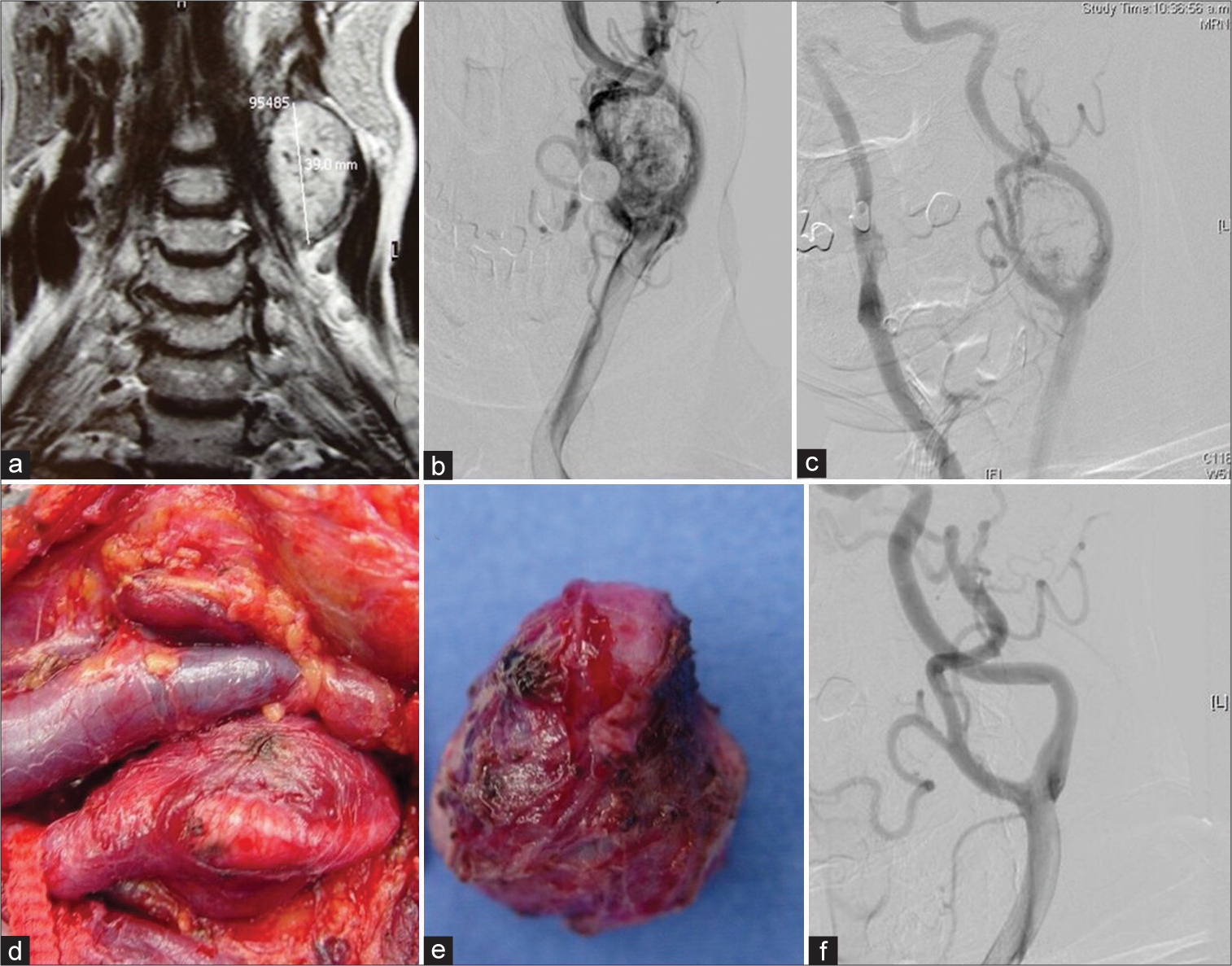
Case 2, Left side CBT, Shamblin II. (a) MRI coronal T2-weighted view. (b) Preoperative DSA showing the rich vascularity of the tumor. (c) After embolization with polyvinyl alcohol particles, there is an excellent reduction in vascularity. (d) Surgical exposure. The internal jugular vein is seen medial to the carotid artery. The tumor is adherent to the bifurcation and the initial portion of the external carotid artery. The internal carotid artery is not visible here. (e) Tumor after extraction from the surgical bed. A reddish color reveals its vascular nature. (f) Postoperative DSA showing no tumor.
Figure 3:
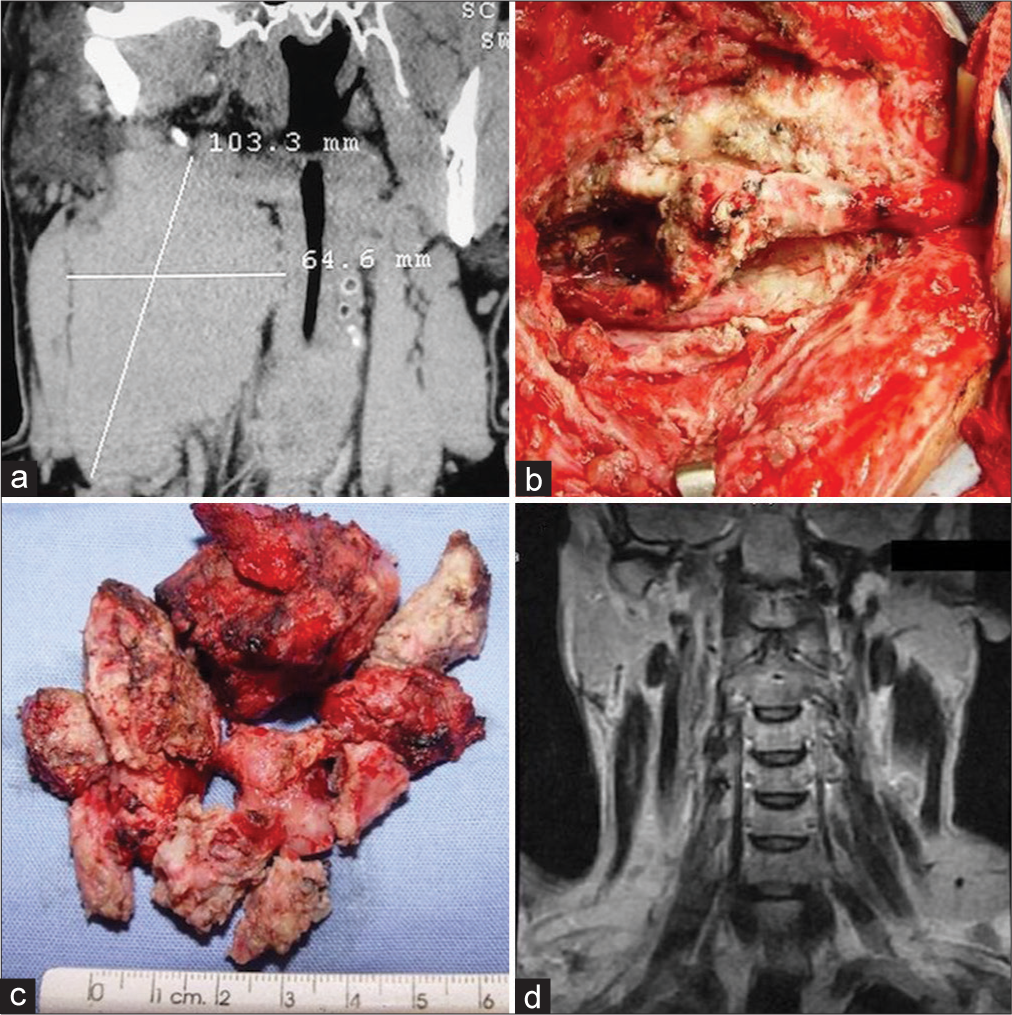
Case 3. Right side CBT, Shamblin III. A 32-year-old male patient had been operated on two different times. In both cases, surgery was stopped due to severe bleeding. (a) CT scan shows the maximum diameters of the lesion and the displacement of the trachea and esophagus. (b) Final view after the tumor was excised under the microscope. The carotid artery is intact after careful dissection. (c) The tumor was obtained in several pieces. No significant bleeding was present during surgery. (d) The control MRI 6 months after surgery shows no residual tumor.
Video 1
RESULTS
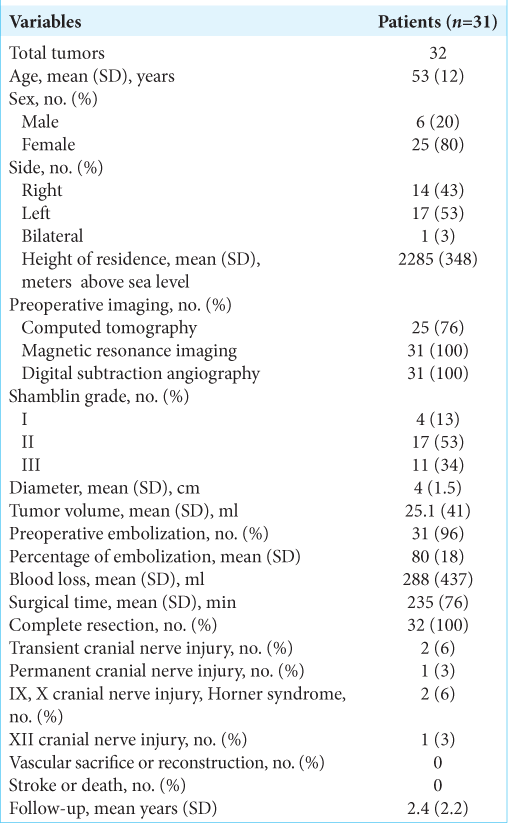
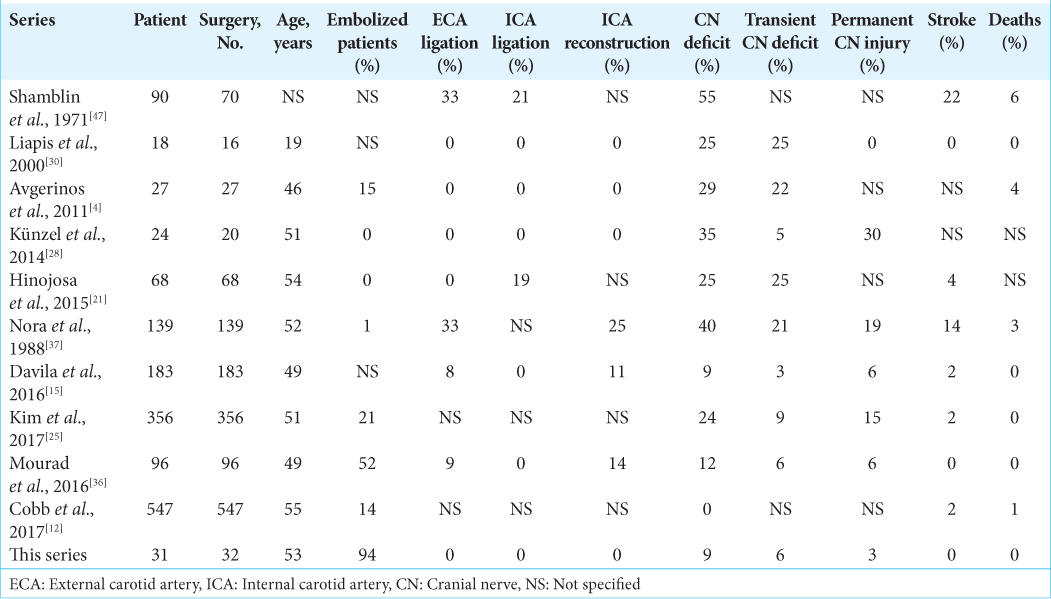
A total of 31 patients involving 32 tumors (80% of women and 20% of men) with a confirmed diagnosis of CBT were included in the study. The mean age was 53 years (SD ± 12 years, range 29–75). All patients were submitted to periadventitial microsurgical resection, as previously described in the surgical technique. Preexisting conditions were present in 42% of patients, including hypertension in 33%, diabetes mellitus type 2 in 9%, and previous vascular disease (stroke and aneurysm) in 9% of patients [
Regarding location, 17 (53%) patients were left sided and one patient presented with bilateral tumors. About 97% of the patients resided at more than 1500 m above sea level (range 1560–2760 m above sea level). The most frequent symptom was the presence of a deep, painless, and slow-growing cervical mass on 23 (74%) patients, followed by vertigo (19%), headache (13%), dysphagia (10%), and others (18%, i.e., odynophagia, tinnitus, and visual disturbances). The mass effect caused by the tumor was associated with dysphagia in three patients. Two patients were asymptomatic, and the tumor was diagnosed by routine testing for nonrelated diseases.
For the diagnostic workout, a CT scan or a CT angiogram was performed on 25 (81%) patients, MRI and DSA were obtained for all patients. Regarding Shambling grade, the most frequent was type II in 53% of the tumors followed by type III in 34% and type I in 13%. The mean maximal diameter of the tumor was 4 cm (SD ± 1.52 cm, range 2–10 cm) with a mean volume of 25 ml (SD ± 40 ml, range 1.26–232.48 ml). In 94% of the patients, preoperative embolization was performed, achieving an average of 80% of decrease in tumoral blood flow. All the patients had complete resection of the tumor, regardless of the Shamblin grade. Transient cranial nerve deficit was observed in 3 (10%) patients in the immediate postoperative period; two patients presented with glossopharyngeal and vagus nerve deficit as well as Horner’s syndrome as a transitory lesion, and one patient presented hypoglossal nerve deficit. None of the patients required carotid sacrifice or vascular reconstruction, regardless of the extent of carotid invasion (Shamblin grade). No patient presented postoperative stroke or death. The average follow-up was 2.4 years (SD ± 2.3 years, range 0.5– 10 years), and at the end of follow-up, only 1 (3%) patient remained with a hypoglossal nerve deficit.
The correlation between Shamblin grade, the diameter of the tumor, tumoral volume, and surgical bleeding was analyzed. We found in the univariate linear regression model that the maximal diameter of the tumor was significantly correlated with the Shamblin grade (r = 0.61, P < 0.001) and the amount of surgical bleeding (r = 0.48, P = 0.005). In contrast, surgical bleeding was not significantly correlated with Shamblin grade (r = 0.29, P = 0.09). Moreover, the univariable linear regression models showed a significant association between the maximal tumoral diameter and the amount of surgical bleeding (r2 = 0.56, P < 0.001), and between tumoral volume and surgical bleeding (r2 = 0.76, P < 0.001).
In addition, we found that every 1 ml of tumoral volume was associated with a 1% increase in risk for a cranial nerve injury (OR 1.02, 95% CI 1.001-1.03, P = 0.046). Notably, we found that tumors with a maximal diameter >5 cm increased the risk for cranial nerve injury by more than 13 times (OR 12.61, 95% 1.25-127.2, P = 0.03). We did not find significant associations between the risk for cranial nerve injury and the Shamblin grade of the tumor (OR 2.9, 95% CI 0.3–33, P = 0.2).
DISCUSSION
Paragangliomas are part of the neuroendocrine tumors that arise from the embryonic neural crest cells.[
As Arya et al. described, “incorporating volume in the classification to predict the Shamblin would have misled the attempt to predict the vascular outcome concerning the ICA.”[
The definitive treatment for CBTs is complete surgical removal with adequate preservation of the neurovascular structures.[
Shamblin et al., in 1971, suggested a surgical classification into three groups. This classification was based on the tumor relationship with the vessel, the intraoperative findings, and postoperative specimen examination, as mentioned above. Shamblin’s classification was used for preoperative risk stratification. According to this classification, the bigger the tumor, the greater the risk of vascular and cranial nerve injury. In their study, more than 5 cm tumor held mortality between 1 and 3% after surgical intervention.[
Schick et al. first introduced the concept of preoperative embolization for CBT resection in 1980.[
Regarding vascular reconstruction and sacrifice, the subadventitial resection aided with the intraoperative Doppler in our cohort allowed us to find a clear interval between the tumor and the carotid artery, avoiding the need for ligating or sacrificing any vessel, even in tumors classified as highly invasive (Shamblin III). Up to the time of publication, we have found three reported series in which no carotid ligation or vascular sacrifice was made,[
Special mention should be made to introducing a microsurgical technique for resection (operating microscope, bipolar forceps, and microinstruments). The present article is the first reported series using this modality consistently, and according to our results, this technique should be encouraged.
Limitations
The study’s retrospective nature and the small number of patients represent a limitation. Nevertheless, the senior author’s experience with an infrequent disease in a large reference center with a high number of carotid artery surgeries confers weight on the collected data.
CONCLUSION
CBTs are rare and often benign tumors presenting as a slow-growing pulsatile cervical mass. They require early diagnosis and multidisciplinary treatment. Imaging studies such as Doppler, MRI, CT, and angiography are essential for an accurate diagnosis. Despite its risks, surgery is the treatment of choice. In our concept, two essential elements introduced in this series have proven to be valuable in decreasing the morbidity and mortality related to this surgery: (1) systematic preoperative embolization confers a significant reduction in blood loss and (2) periadventitial dissection based on a microsurgical technique and delicate management of the vessels and nerves: usage of bipolar coagulation instead of monopolar electrocautery allows a complete resection with no need to perform carotid ligation or reconstruction and reduces the risk of damage to adjacent neural structures.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abu-Ghanem S, Yehuda M, Carmel N, Abergel A, Fliss D. Impact of preoperative embolization on the outcomes of carotid body tumor surgery: A meta-analysis and review of the literature. Head Neck. 2016. 38: E2386-94
2. Amato B, Bianco T, Compagna R, Siano M, Esposito G, Buffone G. Surgical resection of carotid body paragangliomas: 10 years of experience. Am J Surg. 2014. 207: 293-8
3. Arya S, Rao V, Juvekar S, Dcruz A. Carotid body tumors: Objective criteria to predict the Shamblin group on MR imaging. JNR Am J Neuroradiol. 2008. 29: 1349-54
4. Avgerinos ED, Moulakakis K, Brountzos E, Giannakopoulos TG, Lazaris AM, Koumarianou A. Advances in assessment and management of carotid body tumors. Vascular. 2011. 19: 250-6
5. Bakoyiannis CN, Georgopoulos SE, Tsekouras NS, Klonaris CN, Skrapari IC, Papalambros EL. Surgical management of extracranial internal carotid aneurysms by cervical approach. ANZ J Surg. 2006. 76: 612-7
6. Bercin S, Muderris T, Sevil E, Gul F, Kılıcarslan A, Kiris M. Efficiency of preoperative embolization of carotid body tumor. Auris Nasus Larynx. 2015. 42: 226-30
7. Browse N. Carotid body tumours. BMJ. 1982. 284: 1507-8
8. Capatina C, Ntali G, Karavitaki N, Grossman A. The management of head-and-neck paragangliomas. Endocr Relat Cancer. 2013. 20: R291-305
9. Céruse P, Ambrun A, Cosmidis A, Dubreuil C, Feugier P. Paragangliomas laterocervicales. EMC Otorrinolaringol. 2014. 43: 1-16
10. Chapman D, Lippert D, Geer C, Edwards HD, Russell GB, Rees CJ. Clinical, histopathologic, and radiographic indicators of malignancy in head and neck paragangliomas. Otolaryngol Head Neck Surg. 2010. 143: 531-7
11. Chiu G, Edwards A, Akhtar S, Hill J, Hanson I. Carotid body paraganglioma manifesting as a malignant solitary mass on imaging: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010. 109: e54-8
12. Cobb AN, Barkat A, Daungjaiboon W, Halandras P, Crisostomo P, Kuo PC. Carotid body tumor resection: Just as safe without preoperative embolization. Ann Vasc Surg. 2018. 46: 54-9
13. Darouassi Y, Alaoui M, Touati MM, Al Maghraoui O, EnNouali A, Bouaity B. Carotid body tumors: A case series and review of the literature. Ann Vasc Surg. 2017. 43: 265-71
14. Davidovic LB, Djukic VB, Vasic DM, Sindjelic RP, Duvnjak SN. Diagnosis and treatment of carotid body paraganglioma: 21 years of experience at a clinical center of Serbia. World J Surg Oncol. 2005. 3: 10
15. Davila VJ, Chang JM, Stone WM, Fowl RJ, Bower TC, Hinni ML. Current surgical management of carotid body tumors. J Vasc Surg. 2016. 64: 1703-10
16. Druck N, Spector G, Ciralsky R, Ogura J. Malignant glomus vagale: Report of a case and review of the literature. Arch Otolaryngol Head Neck Surg. 1976. 102: 634-6
17. Frey C, Karoll R. Management of chemodectomas. Am J Surg. 1966. 111: 536-42
18. Gordon-Taylor G. On carotid tumours. Br J Surg. 1940. 28: 163-72
19. Heath D, Edwards C, Harris P. Post-mortem size and structure of the human carotid body: Its relation to pulmonary disease and cardiac hypertrophy. Thorax. 1970. 25: 129-40
20. Heath D. The human carotid body. Thorax. 1983. 38: 561-564
21. Hinojosa CA, Ortiz-Lopez LJ, Anaya-Ayala JE, OrozcoSevilla V, Nunez-Salgado AE. Comparison of retrocarotid and caudocranial dissection techniques for the surgical treatment of carotid body tumors. J Vasc Surg. 2015. 62: 958-64
22. Irwin G. Carotid body tumors. Am J Roentgenol. 1965. 95: 769-74
23. Jackson R, Myhill J, Padhya T, McCaffrey J, McCaffrey T, Mhaskar R. The effects of preoperative embolization on carotid body paraganglioma surgery. Otolaryngol Head Neck Surg. 2015. 153: 943-50
24. Kasper G, Welling R, Wladis A, CaJacob DE, Grisham AD, Tomsick TA. A multidisciplinary approach to carotid paragangliomas. Vasc Endovasc Surg. 2007. 40: 467-74
25. Kim GY, Lawrence PF, Moridzadeh RS, Zimmerman K, Munoz A, Luna-Ortiz K. New predictors of complications in carotid body tumor resection. J Vasc Surg. 2017. 65: 1673-9
26. Knight TT, Gonzalez JA, Rary JM, Rush DS. Current concepts for the surgical management of carotid body tumor. Am J Surg. 2006. 191: 104-10
27. Kruger A, Walker P, Foster W, Jenkins J, Boyne N, Jenkins J. Important observations made managing carotid body tumors during a 25-year experience. J Vasc Surg. 2010. 52: 1518-23
28. Künzel J, Koch M, Brase C, Fietkau R, Iro H, Zenk J. Treatment of cervical paragangliomas: Is surgery the only way?. Am J Otolaryngol. 2014. 35: 186-91
29. Lee J, Barich F, Karnell L, Robinson RA, Zhen WK, Gantz BJ. National cancer data base report on malignant paragangliomas of the head and neck. Cancer. 2002. 94: 730-7
30. Liapis CD, Evangelidakis EL, Papavassiliou VG, Kakisis JD, Gougoulakis AG, Polyzos AK. Role of malignancy and preoperative embolization in the management of carotid body tumors. World J Surg. 2000. 24: 1526-30
31. Lim J, Kim J, Kim S, Lee S, Lim YC, Kim JW. Surgical treatment of carotid body paragangliomas: Outcomes and complications according to the Shamblin classification. Clin Exp Otorhinolaryngol. 2010. 3: 91
32. Litle VR, Reilly LM, Ramos TK. Preoperative embolization of carotid body tumors: When is it appropriate?. Ann Vasc Surg. 1996. 10: 464-8
33. Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Granados-Garcia M, Herrera-Gomez A. Carotid body tumors: Review of a 20-year experience. Oral Oncol. 2005. 41: 56-61
34. Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Herrera-Gomez A. Does Shamblin’s classification predict postoperative morbidity in carotid body tumors? A proposal to modify Shamblin’s classification. Eur Arch Otorhinolaryngol. 2006. 263: 1161
35. Monro R. The natural history of carotid body tumours and their diagnosis and treatment. With a report of five cases. Br J Surg. 1950. 37: 445-53
36. Mourad M, Saman M, Stroman D, Brown R, Ducic Y. Evaluating the role of embolization and carotid artery sacrifice and reconstruction in the management of carotid body tumors. Laryngoscope. 2016. 126: 2282-7
37. Nora JD, Hallett JW, O’Brien PC, Naessens JM, Cherry KJ, Pairolero PC. Surgical resection of carotid body tumors: Long-term survival, recurrence, and metastasis. Mayo Clin Proc. 1988. 63: 348-52
38. Oosterwijk J, Jansen J, van Schothorst E, Oosterhof AW, Devilee P, Bakker E. First experiences with genetic counselling based on predictive DNA diagnosis in hereditary glomus tumours (paragangliomas). J Med Genet. 1996. 33: 379-83
39. Ozyer U, Harman A, Yildirim E, Aytekin C, Akay T, Boyvat F. Devascularization of head and neck paragangliomas by direct percutaneous embolization. Cardiovasc Intervent Radiol. 2010. 33: 967-75
40. Pellitteri P, Rinaldo A, Myssiorek D, Jackson CG, Bradley PJ, Devaney KO. Paragangliomas of the head and neck. Oral Oncol. 2004. 40: 563-75
41. Power A, Bower T, Kasperbauer J, Link MJ, Oderich G, Cloft H. Impact of preoperative embolization on outcomes of carotid body tumor resections. J Vasc Surg. 2012. 56: 979-89
42. Roden D, Myssiorek D. Neck management in malignant head and neck paragangliomas. Oper Tech Otolaryngol Head Neck Surg. 2016. 27: 41-6
43. Rodríguez-Cuevas S, López-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck. 1998. 20: 374-78
44. Sajid MS, Hamilton G, Baker DM. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007. 34: 127-30
45. Sarrat-Torres MA, Torres A, Whyte J, Baena S, Cisneros A, Sarrat R. Structure, location, function and pathological features of the human carotid body. Eur J Anat. 2006. 10: 1-5
46. Schick PM, Hieshima GB, White RA, Fiaschetti FL, Mehringer CM, Grinnell VS. Arterial catheter embolization followed by surgery for large chemodectoma. Surgery. 1980. 87: 459-64
47. Shamblin WR, ReMine WH, Sheps SG, Harrison EG. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971. 122: 732-9
48. Ünlü Y, Becit N, Ceviz M, Koçak H. Treatment of carotid body tumors and familial paragangliomas: A review of 30 years of experience. Ann Chir Vas. 2009. 23: 667-72
49. Van der Mey A, Jansen J, Van Baalen J. Management of carotid body tumors. Otolaryngol Clin North Am. 2001. 34: 907-24
50. Williams MD, Tischler AS, editors. Update from the 4th edition of the world health organization classification of head and neck tumours: Paragangliomas. Head Neck Pathol. 2017. 11: 88-95
51. Woolen S, Gemmete J. Paragangliomas of the head and neck. Neuroimaging Clin N Am. 2016. 26: 259-78
52. Zeitler DM, Glick J, Har-El G. Preoperative embolization in carotid body tumor surgery: Is it required?. Ann Otol Rhinol Laryngol. 2010. 119: 279-83
53. Zhang T, Jiang W, Li Y, Li B, Yamakawa T. Perioperative approach in the surgical management of carotid body tumors. Ann Vasc Surg. 2012. 26: 775-82